CONCEPT14.4 Changes in Gene Expression Pathways Underlie the Evolution of Development
The discovery of the genes that control the development of Drosophila provided biologists with tools to investigate the development of other organisms. For example, when scientists used homeobox DNA as a hybridization probe (see Figure 10.7) to search for similar genes elsewhere, they found that the homeobox is present in many genes in many other organisms. This, and other astounding discoveries that followed, showed a similarity in the molecular events underlying morphogenesis in organisms ranging from flies to fish to mammals. These results suggested that just as the forms of organisms evolved through descent with modification from a common ancestor, so did the molecular mechanisms that produce those forms. Biologists started to ask new questions about the interplay between evolutionary and developmental processes—a field of study called evolutionary developmental biology (evo-devo). The major findings of evo-devo are:
- Organisms share similar molecular mechanisms for development, including a “toolkit” of regulatory molecules that control the expression of genes.
- These regulatory molecules are able to act independently in different tissues and regions of the body, so that evolutionary change can occur in independent “modules.”
- Developmental differences can arise from changes in the timing of action of a regulatory molecule, the location of its action, or the quantity of its action.
- Developmental changes can arise from environmental influences on developmental processes.
The development of a multicellular organism from a fertilized egg—a single cell—involves an intricate pattern of sequential gene expression. When developmental biologists began to describe the events responsible for the differentiation and controlled proliferation of cells and tissues at the molecular level, they found common regulatory genes and pathways in organisms that don’t appear similar at all, such as fruit flies and mice.
Developmental genes in distantly related organisms are similar
Initially through hybridization with a homeobox probe, and then by genome sequencing and comparative genomics (see Concept 12.2), biologists have found that diverse animals share numerous molecular pathways that govern gene expression during development. For example, fruit fly homeotic genes such as Antennapedia and bithorax are similar to mouse (and human) genes that play similar developmental roles. This means that the positional information controlled by these genes has been conserved, even as the structures formed at each position have changed. Over the millions of years that have elapsed since these animals diverged from a common ancestor, the genes in question have mostly been maintained, suggesting that their functions are essential for animal development.
Changes in gene expression pathways underlie the evolution of development
The control of eye formation during the development of many animals is under the control of a genetic switch involving a transcription factor. Partial DNA sequences for the control gene from two organisms are given below.
Mouse Pax6 gene:
5′-GTA TCC AAC GGT TGT GTG AGT AAA ATT-3′
Fruit fly eyeless gene:
5′-GTA TCA AAT GGA TGT GTG AGC AAA ATT-3′
- Calculate the percentage of identity (the percentage of bases that are identical) between the two DNA sequences.
- Use the genetic code (see Figure 10.11) to determine the amino acid sequences (see Table 3.2) encoded by the two regions and calculate the percentage of identity between the two amino acid sequences.
- The fruit fly and the mouse diverged from a common ancestor about 500 million years ago. Comment on your answers to 1 and 2 in terms of the evolution of developmental pathways.
Remarkably, these genes are arranged along a chromosome in both the fruit fly and mouse in the same order as they are expressed along the anterior–posterior axis of their embryos (FIGURE 14.15). In the mouse and other vertebrates, these genes are switched on sequentially during embryogenesis. The Hox genes controlling the development of anterior structures (such as the head) are expressed earliest, and as a result, anterior structures develop earlier than posterior structures. The spatial organization of the Hox genes on the chromosome is important for the timing of expression of the genes.
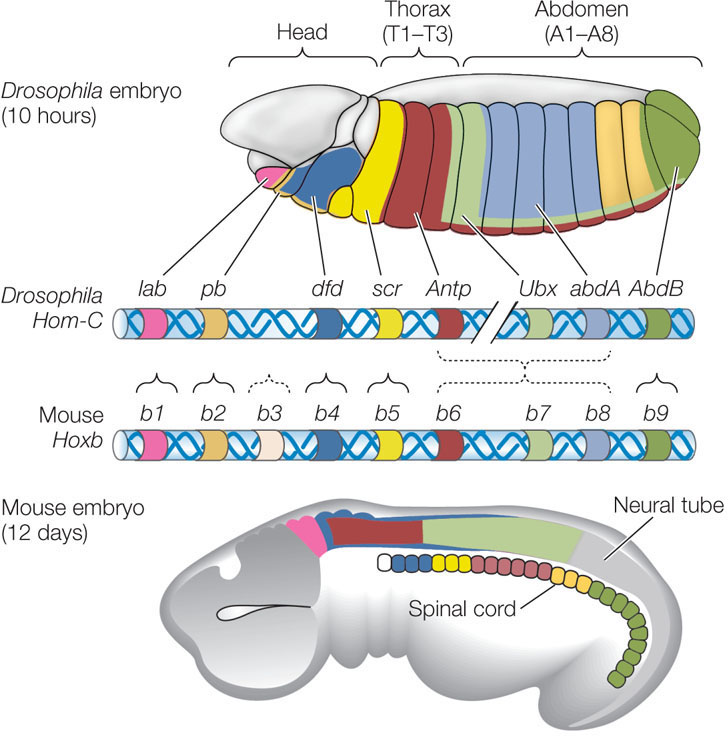
These and other examples have led biologists to the idea that certain developmental mechanisms, controlled by specific DNA sequences, have been conserved over long periods during the evolution of multicellular organisms. These sequences comprise a genetic toolkit, the contents of which have been modified and reshuffled over the course of evolution to produce the remarkable diversity of plants, animals, and other organisms in the world today.
Genetic switches govern how the genetic toolkit is used
The genetic toolkit is also used to generate diverse structures within a single organism. Different structures can evolve within a single organism using a common set of genetic instructions because there are mechanisms called genetic switches (also called molecular switches) that control how the genetic toolkit is used. As we have seen, these mechanisms involve promoters and transcription factors. The signal cascades that converge on and operate these switches determine when and where genes will be turned on and off. Multiple switches control each gene by influencing its expression at different times and in different places. In this way, elements of the genetic toolkit can be involved in multiple developmental processes while still allowing individual modules to develop independently. For example, the morphogenesis of different flower organs is determined by different combinations of three classes of transcription factors (the ABC model; see Figure 14.12) acting at specific times and locations.
During evolution, changes in the functions of genetic switches have led to changes in the forms or functions of organisms. To illustrate this, let’s look at the development of wings in Drosophila and other insects. Drosophila species are members of the insect group Diptera, which means “two wings”—that is, they have a single pair of wings, whereas most insects have two pairs of wings (i.e., four wings). In dipterans, the single pair of wings develops on the second thoracic segment, and a pair of balancing organs called halteres develops on the third thoracic segment. A critical difference between thoracic segments 2 and 3 is that the Hox gene Ultrabithorax (Ubx) is expressed in segment 3 but not in segment 2. The Ubx transcription factor represses the development of wings in dipterans (FIGURE 14.16). If the Drosophila Ubx gene is inactivated by mutation, a second pair of wings forms in thoracic segment 3. In other insects such as butterflies, Ubx turns on the expression of wing-forming genes so that full hindwings develop. Therefore a simple genetic change in the effect of Ubx on genes that promote wing development results in a major morphological difference in the wings of flies and butterflies. This phenomenon—the same switch having different effects on target genes in different species—is important in evolution.
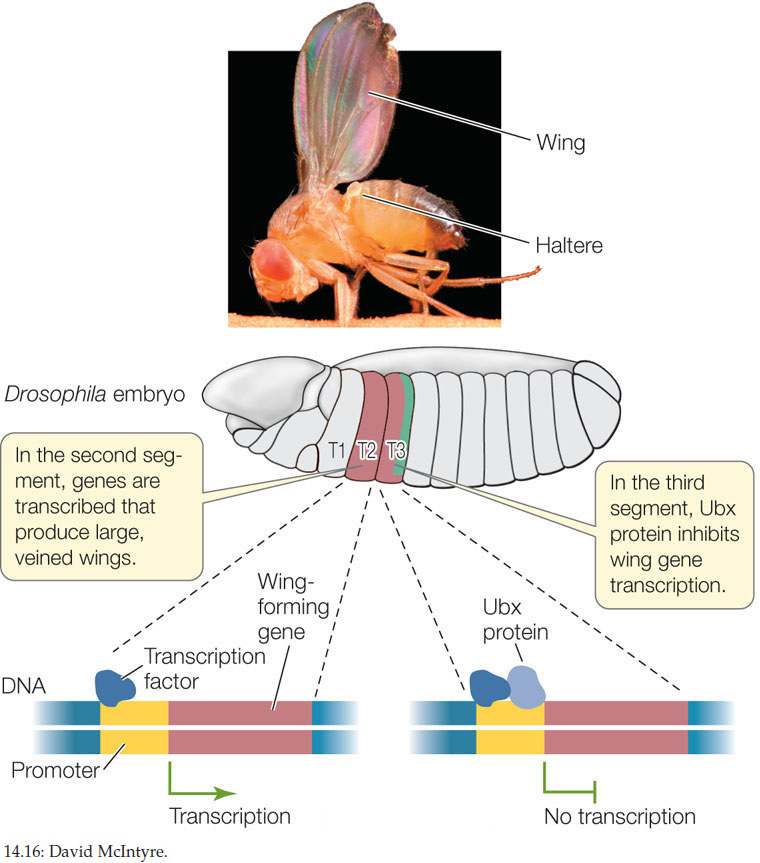
Modularity allows for differences in the pattern of gene expression among organisms
The modularity of development means that the molecular pathways for developmental processes such as organ formation operate independently from one another. For example, an Antennapedia mutant grows a leg where an antenna should be (see Figure 14.14), but all of the mutant’s other organs develop normally and in their proper places. On an evolutionary time scale, modularity means that the timing and position of a particular developmental process can change without disrupting the whole organism.
Timing Differences
The genes regulating the development of a module may be expressed at different developmental stages or for different durations in different species, a phenomenon called heterochrony. An example is the evolution of the giraffe’s neck. As in virtually all mammals (with the exception of manatees and sloths), there are seven vertebrae in the neck of the giraffe. So the giraffe did not get a longer neck than other mammals by adding vertebrae. Instead, each of the cervical (neck) vertebrae of the giraffe is much longer than those of other mammals (FIGURE 14.17). How does this happen?
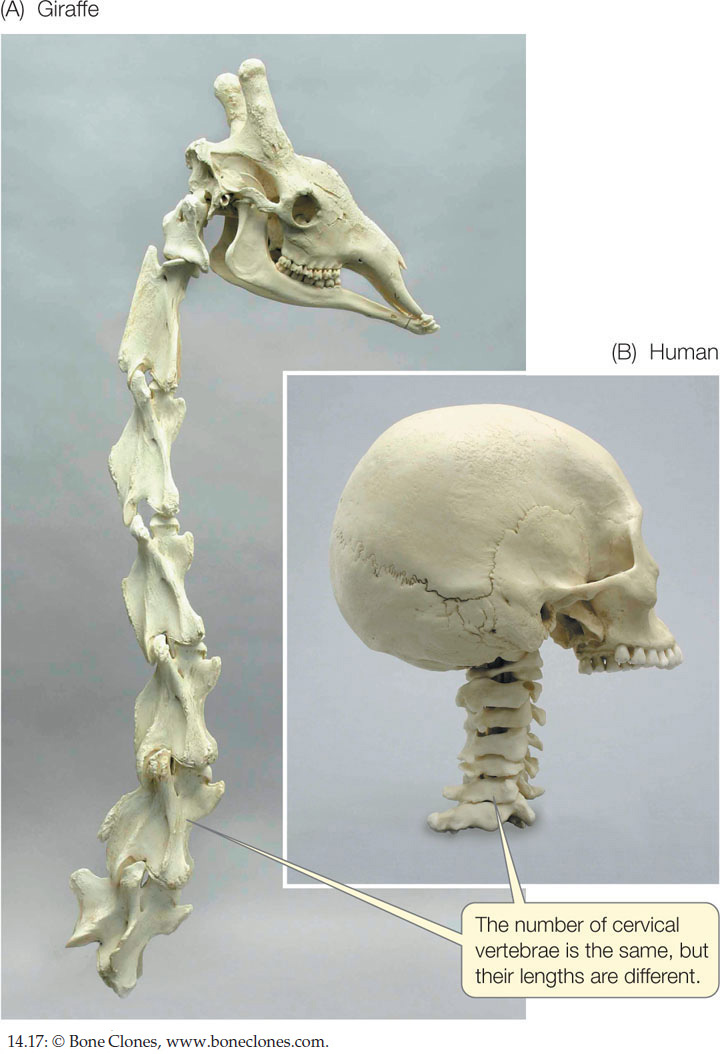
Bones grow because of the proliferation of cartilage-producing cells called chondrocytes. Bone growth is stopped by a signal that results in death of the chondrocytes and the accumulation of calcium salts in the bone. In giraffes this signaling process is delayed in the cervical vertebrae, with the result that these vertebrae grow longer. Thus the evolution of longer necks occurred through changes in the timing of expression of the genes that control bone formation.
Spatial Differences
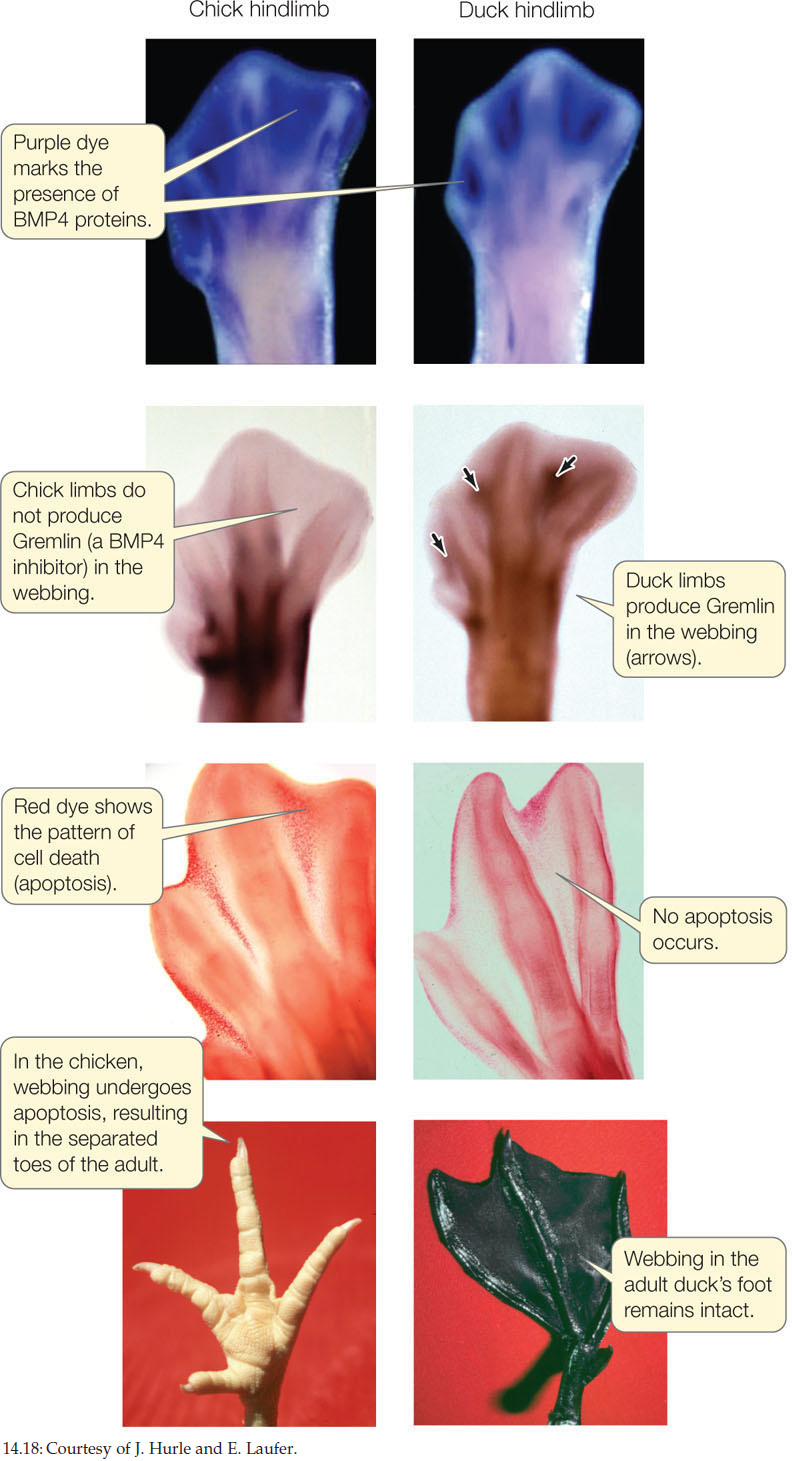
Changes in the spatial expression pattern of a developmental gene are known as heterotopy and can also result in evolutionary change. For example, the difference in foot webbing in ducks versus chickens is determined by an alteration in the spatial expression of a single gene. The feet of all bird embryos have webs of skin that connect their toes. This webbing is retained in adult ducks (and other aquatic birds) but not in adult chickens (and other non-aquatic birds). The loss of webbing is caused by a signaling protein called bone morphogenetic protein 4 (BMP4) that instructs the cells in the webbing to undergo apoptosis. The death of these cells destroys the webbing between the toes.
Embryonic duck and chicken hindlimbs both express the BMP4 gene in the webbing between the toes, but they differ in expression of a gene called Gremlin, which encodes a BMP inhibitor protein (FIGURE 14.18). In ducks, but not chickens, the Gremlin gene is expressed in the webbing cells. The Gremlin protein inhibits the BMP4 protein from signaling for apoptosis, and the result is a webbed foot.
CHECKpointCONCEPT14.4
- Describe the major ideas of evolutionary developmental biology.
- What is the evidence that there was a common origin for the developmental pathways leading to segment identity in insects and the organization of the spinal cord in mice?
- What is the evidence that changes in the transcription of a single gene can lead to differences in morphogenesis between different regions of developing organ?
- Examine Figure 14.18 and the related text. If Gremlin protein were added to the webbed region between the developing toes of a chicken, what would be the result?
We have seen how the genetic toolkit guides morphogenesis in individual organisms, and how differences in genetic switches contribute to differences among species. In the next concept we will discuss further the roles that some of these same tools play in the evolution of new forms and new species.