Concept 25.3: Water and Solutes Are Transported in the Xylem by Transpiration–Cohesion–Tension
Plants require water to carry out photosynthesis, to transport solutes between plant organs, to cool their leaves by evaporation, and to maintain the internal pressure that supports their bodies. Here we focus on how water moves into and out of plant cells and between different parts of the plant.
Differences in water potential govern the direction of water movement
Water moves into and out of plant cells by osmosis. In plant cells, the direction of water movement is determined by water potential (psi, ψ), defined as the tendency of a solution (water plus solutes) to take up water from pure water across a membrane. Whenever water moves across a selectively permeable membrane by osmosis, it moves toward the region of lower (more negative) water potential (FIGURE 25.8A, LEFT). Water potential is expressed in megapascals (MPa), a unit of pressure. Atmospheric pressure—“one atmosphere”—is about 0.1 MPa, or 14.7 pounds per square inch (a typical pressure in an automobile tire is about 0.2 MPa).
LINK
You can review the structure and function of the cell membrane in Chapter 5; Concept 5.2 describes osmosis
Water potential has two components:
- Solute potential (ψs): As solutes are added to pure water, the concentration of free water is reduced, the tendency to take up water increases, and water potential decreases. (In terms of cells, as solutes are added to the environment, it becomes hypertonic relative to the cells.)
- Pressure potential (ψp): When any closed compartment takes up water, it tends to swell. The walls of the compartment, however, resist that swelling (think of blowing up a balloon). The result is an increase in internal pressure in the compartment, which decreases the tendency to take up more water (increases the water potential; FIGURE 25.8A, RIGHT).
 Figure 25.8: Water Potential, Solute Potential, and Pressure Potential (A) A theoretical illustration of water potential. (B) The effect of differences in water potential on a plant cell.
Figure 25.8: Water Potential, Solute Potential, and Pressure Potential (A) A theoretical illustration of water potential. (B) The effect of differences in water potential on a plant cell.
Go to ANIMATED TUTORIAL 25.2 Water Uptake in Plants
PoL2e.com/at25.2
We can express the effects of solute potential and pressure potential on water potential in the form of an equation:
ψ = ψs + ψp
By definition the solute potential of pure water is zero; because added solutes decrease water potential, solute potential is usually negative. Pressure potential is defined as zero when it equals atmospheric pressure. Pressure potentials less than atmospheric pressure are negative, and those greater than atmospheric pressure are positive.
In a plant cell (FIGURE 25.8B), the cell wall resists the swelling that would otherwise occur as the cell takes up water (see also Figure 5.3C). The resulting turgor pressure is equivalent to the pressure potential exerted by the piston in Figure 25.8A. Water will enter plant cells by osmosis until the pressure potential exactly balances the solute potential. At this point the cell is said to be “turgid”—that is, it has a significant positive pressure potential. The physical structure of many plants is maintained by the (positive) pressure potential of their cells; if the pressure potential drops (for example, if the plant does not have enough water), the plant wilts (FIGURE 25.9).

546
Water and ions move across the root cell’s cell membrane
The movement of a soil solution containing mineral ions across a root cell’s cell membrane faces two major challenges:
- The membrane is hydrophobic, whereas water and mineral ions are polar.
- Some mineral ions must be moved against their concentration gradients.
These two challenges are met by the activities of membrane proteins.
Aquaporins
Aquaporins are membrane channels through which water can diffuse (see Figure 5.5). Although aquaporins were first discovered in animal cells, they are also abundant in plant cell membranes. The abundance and activity of aquaporins are both regulated so that the rate of osmosis can be controlled. But the direction of osmosis is always the same: water moves to the region of more negative water potential.
Ion Channels and Proton Pumps
When the concentration of an ion in the soil solution is greater than that inside the root cells, transport proteins can move the ion into the root cells by facilitated diffusion. The concentrations of many mineral ions in the soil solution, however, are lower than those inside the plant. In addition, electric charge differences play a role in the uptake of mineral ions. For example, a negatively charged ion that moves into a negatively charged cellular compartment is moving against an electrical gradient, and this movement requires energy. Concentration and electrical gradients combine to form an electrochemical gradient. Uptake against an electrochemical gradient requires active transport.
547
LINK
Electrochemical gradients are discussed in more detail in Concept 34.2
Animals use a Na+−K+ pump to generate a Na+ gradient that can be used to drive the uptake of other solutes (see Figure 5.7). Instead of a Na+−K+ pump, plants have a proton pump, which uses energy from ATP to move protons out of the cell against a proton concentration gradient (FIGURE 25.10, STEP 1). Because protons (H+) are positively charged, their accumulation outside the cell has two results:
- An electrical gradient is created such that the region just outside the cell becomes more positively charged than the inside of the cell.
- A proton concentration gradient develops, with more protons just outside the cell than inside the cell.

With the inside of the cell more negative than the outside, cations such as potassium (K+) move into the cell down the electrical gradient through specific membrane channels (FIGURE 25.10, STEP 2). Anions such as chloride (Cl−) are moved into the cell against an electrochemical gradient by a membrane transport protein that couples their movement with that of H+ (FIGURE 25.10, STEP 3). These processes are examples of secondary active transport.
Water and ions pass to the xylem by way of the apoplast and symplast
The journey of water and ions from the soil through the roots to the xylem follows two pathways—a fast lane (the apoplast) and a slower lane (the symplast)—either separately or in tandem:

- The apoplast (Greek apo, “away from”; plast, “living material”) consists of the cell walls, which lie outside the cell membranes, and intercellular spaces (spaces between cells), which are common in many plant tissues. The apoplast is a continuous meshwork through which water and solutes can flow without ever having to cross a membrane.
- The symplast (Greek sym, “together with”) passes through the continuous cytoplasm of the living cells connected by plasmodesmata. The selectively permeable cell membranes of the root cells control access to the symplast, so movement of water and solutes into the symplast is tightly regulated.
Water and minerals that pass from the soil solution through the apoplast can travel freely as far as the endodermis, the innermost layer of the root cortex (FIGURE 25.11).

Go to ACTIVITY 25.1 Apoplast and Symplast of the Root
PoL2e.com/ac25.1
The endodermis is distinguished by the presence of the Casparian strip. This waxy, suberin-impregnated region of the endodermal cell wall forms a hydrophobic belt around each endodermal cell where it is in contact with other endodermal cells. The Casparian strip acts as a seal that prevents water and ions from moving through spaces between the endodermal cells; instead, water and ions enter the cytoplasm of the endodermal cells. Water and ions already in the symplast can enter the endodermal cells through plasmodesmata.
548
LINK
The Casparian strip is functionally equivalent to the tight junctions found in some animal tissues; see Figure 4.18
Once they have passed the endodermal barrier, water and minerals remain in the symplast until they reach parenchyma cells in the pericycle or xylem. These cells then actively transport mineral ions into the apoplast of the stele. As ions are transported into the solution in the cell walls of the stele, water potential in the apoplast becomes more negative; consequently, water moves out of the cells and into the apoplast by osmosis. In other words, ions are transported actively, and water follows passively. The end result is that water and minerals end up in the xylem, where they constitute the xylem sap.
Water moves through the xylem by the transpiration–cohesion–tension mechanism
Once water has arrived in the xylem, it is all uphill from there! Consider the magnitude of what xylem accomplishes. A single maple tree 15 meters tall has been estimated to have some 177,000 leaves, with a total leaf surface area of 675 square meters—1.5 times the area of a basketball court. During a summer day, that tree loses 220 liters of water per hour to the atmosphere by evaporation from its leaves. To prevent wilting, the xylem must transport 220 liters of water 15 meters from the roots up to the leaves every hour.
How is the xylem able to move so much water to such great heights? Part of the answer lies in the structure of the xylem, which we described in Chapter 24. Recall that xylem vessels consist of a long “straw” of cell walls, which provide both structural support and the rigidity needed to maintain a pressure gradient within the xylem sap. Early experiments ruled out two possible ways that had been hypothesized for moving water up through these vessels from roots to leaves: upward pressure and capillary action.
Upward pressure would move water by “pushing the bottom.” A simple experiment in 1893 ruled out this mechanism: A tree was cut at its base, and the sawed-off upper stem was placed in a vat of poison that killed living cells. (Recall that mature xylem is made up of dead cells.) The poison continued to rise through the trunk, even though cells were killed along the way. This observation showed that a living “pump” is not required to push the xylem sap up a tree. Furthermore, the roots were absent, yet upward flow in the trunk continued, demonstrating that the roots do not provide upward pressure. When the poison reached the leaves and killed them, however, all further movement up the trunk stopped, demonstrating that leaves must be alive for water to move in the xylem.
Capillary action was also ruled out as the mechanism that makes water rise in the xylem. Water molecules have strong cohesion, so a column of water can indeed rise a short distance in a thin tube (e.g., a straw or a xylem vessel) by capillary action, but calculations have shown that xylem vessels, at 100 μm in diameter, are simply too wide to get water to the top of a 15-meter tree in this fashion. In fact, the maximum height for a water column raised by capillary action alone in a 100-μm-diameter vessel is only 0.15 meters.
549
LINK
You can review the properties of water in Concept 2.2
Almost a century ago, the transpiration–cohesion–tension theory was proposed to explain water transport in the xylem (FIGURE 25.12). According to this theory, the key elements of xylem transport are:
- Transpiration: evaporation of water from cells within the leaves
- Cohesion of water molecules in the xylem sap as a result of hydrogen bonding
- Tension on the xylem sap resulting from transpiration


Go to ANIMATED TUTORIAL 25.3 Xylem Transport
PoL2e.com/at25.3

Go to MEDIA CLIP 25.1 Inside the Xylem
PoL2e.com/mc25.1
The concentration of water vapor in the atmosphere is lower than that in the air spaces between the cells of a leaf. Because of this difference, water vapor diffuses out of the leaf in a process called transpiration. Within the leaf, water evaporates from the moist walls of the mesophyll cells and enters the intercellular air spaces. As water evaporates from the aqueous film coating each cell, the film shrinks back into tiny spaces in the cell walls, increasing the curvature of the water surface and thus increasing its surface tension. Because of hydrogen bonding, water molecules have cohesion, and therefore the increased tension (negative pressure potential) in the surface film draws more water into the cell walls, replacing that which was lost by evaporation. The resulting tension in the mesophyll draws water from the xylem of the nearest vein into the apoplast surrounding the mesophyll cells. The removal of water from the veins, in turn, establishes tension on the entire column of water contained in the xylem, so that the column is drawn upward all the way from the roots.
550
Each part of this model is supported by evidence:
- The difference in water potential between the soil solution and air is huge, on the order of −100 MPa. This difference generates more than enough tension to pull a water column up the tallest tree.
- There is a continuous column of water in the xylem, which is caused by cohesion.
- Measurements of xylem pressures in cut stems show a negative pressure potential, which indicates considerable tension.
In addition to its role in xylem transport, transpiration has the added benefit of cooling a plant’s leaves (much as humans sweat to cool off). The evaporation of water from mesophyll cells consumes heat, thereby decreasing the leaf temperature. A farmer can hold a leaf between thumb and forefinger to estimate its temperature; if the leaf doesn’t feel cool, that means transpiration is not occurring and it must be time to water.
Although transpiration provides the driving force for the transport of water and minerals in the xylem, it also results in the loss of tremendous quantities of water from the plant. How do plants control this loss?
Stomata control water loss and gas exchange
In Chapter 6 we described photosynthesis, which has the general equation
CO2 + H2O → carbohydrate + O2
Leaves are the chief organs of photosynthesis, so they must have a large surface area across which to exchange the gases O2 and CO2. This surface area is provided by the mesophyll cells being surrounded by abundant air spaces inside the leaf (see Figure 25.12), which are also the location of the evaporation that drives water movement in the xylem. The epidermis of leaves and stems minimizes transpirational water loss by secreting a waxy cuticle, which is impermeable to water. However, the cuticle is also impermeable to CO2 and O2. How can the plant balance its need to retain water with its need to obtain CO2 for photosynthesis?
Plants have met this challenge with the evolution of stomata (singular stoma): pores in the leaf epidermis, typically on the underside of the leaf (FIGURE 25.13A). There are up to 1,000 stomata per square millimeter in some leaves, constituting up to 3 percent of the total surface area of the shoot. Such an abundance of stomata could lead to excessive water loss. But stomata are not static structures. A pair of specialized epidermal cells, called guard cells, controls the opening and closing of each stoma.
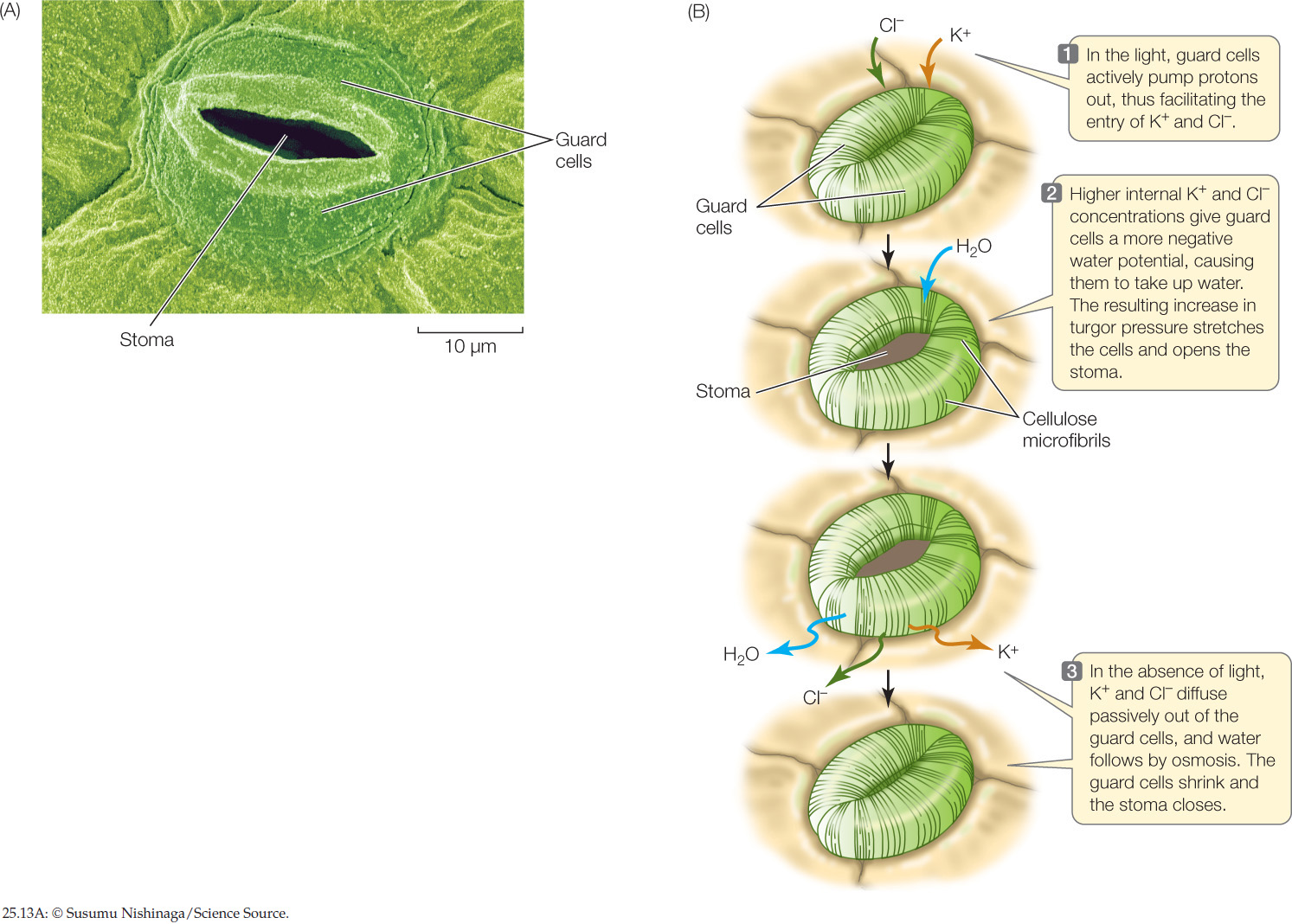
When the stomata are open, CO2 can enter the leaf by diffusion, but water vapor diffuses out of the leaf at the same time. Closed stomata prevent water loss but also exclude CO2 from the leaf. Most plants open their stomata when the light intensity is sufficient to maintain a moderate rate of photosynthesis. At night, when darkness precludes photosynthesis, their stomata are closed; no CO2 is needed at this time, and water is conserved. Even during the day, the stomata close if water is being lost at too great a rate.
551
APPLY THE CONCEPT: Water and solutes are transported in the xylem by transpiration–cohesion–tension
Suppose you measure water potential (ψ) in a 100-m-tall tree and its surroundings and obtain the results in the table. Note that gravity exerts a force of −0.01 MPa per meter of height above ground.
- Is the water potential in the leaf sufficiently low to draw water to the top of the tree?
- Would transpiration continue if soil water potential decreased to −1.0 MPa?
- What would you expect to happen to the xylem water potential if all of the stomata closed?

Stomata can respond rapidly to changes in light, CO2 concentration, and water loss. Plants can also change their total number of stomata in response to longer-term changes in environmental conditions.
Light and Co{sub}2{/sub} Concentration
Guard cells can respond to changes in light and CO2 concentration in a matter of minutes by changing their solute potential. The absorption of light by a pigment in the guard cell’s cell membrane activates a proton pump (see Figure 25.10), which actively transports H+ out of the guard cells and into the apoplast of the surrounding epidermis. The resulting electrochemical gradient drives K+ into the guard cells, where it accumulates (FIGURE 25.13B). Negatively charged chloride (Cl−) ions and organic ions also move into and out of the guard cells along with the K+ ions, maintaining electrical balance. The increased concentration of K+ and other solutes inside the guard cells makes the solute potential of the guard cells more negative. Water then enters by osmosis, increasing the turgor pressure of the guard cells. The guard cells change their shape, becoming more turgid in response to the increase in pressure potential, so that a space—the stoma—appears between them. The stoma closes in the absence of light: the proton pump becomes less active, K+ ions diffuse passively out of the guard cells, water follows by osmosis, the pressure potential decreases, and the guard cells sag together and seal off the stoma. Guard cell membranes are particularly rich in aquaporins, making guard cells well adapted for the rapid water movements involved in stomatal responses.
Water
Stomata also respond to water availability. Water stress is a common problem for plants, especially on a hot, windy day, when plants might close their stomata even when the sun is shining. The water potential of the leaf’s mesophyll cells is the cue for this response. If the mesophyll becomes dehydrated, its cells release the hormone abscisic acid, which causes the stomata to close.
Regulation By Stomata Number
A plant can regulate water loss not just by the opening or closing of stomata, but by changing the number of stomata. This occurs much more slowly, over a period of days to weeks. Trees can reduce stomata numbers by shedding leaves, or by making new leaves that have few stomata.
CHECKpoint CONCEPT 25.3
- What distinguishes water potential, solute potential, and pressure potential?
- What are the roles of transpiration, cohesion, and tension in xylem transport?
- If Arabidopsis is exposed to high CO2 concentrations, the new leaves that form on the plant have fewer stomata than they would have had under normal conditions. Why do you think this might be advantageous?
Now that we understand how leaves obtain the water and CO2 required for photosynthesis, let’s examine how the products of photosynthesis are transported to other parts of the plant where they are needed.