Concept 26.2: Gibberellins and Auxin Have Diverse Effects but a Similar Mechanism of Action
The discovery of two key plant hormones exemplifies the experimental approaches that plant biologists have used to investigate the mechanisms of plant development. Gibberellins (of which there are several active forms) and auxin were the first plant hormones to be identified, early in the twentieth century. In both cases, the discoveries came from observations of natural phenomena:
- Gibberellins: In rice plants, a disease caused by the fungus Gibberella fujikuroi resulted in plants that grew overly tall and spindly.
- Auxin: Charles Darwin and his son Francis noted that canary grass seedlings would bend toward the light when placed near a light source.
In both cases, a chemical substance was isolated that could cause the phenomenon:
- Gibberellic acid (see Table 26.2) made by the fungus caused rice plants to overgrow. Later it was found that plants also make gibberellic acid, and that applying it to plants caused growth.
- Auxin (indole-3-acetic acid) applied asymmetrically to the growing tips of seedlings caused cell expansion on the side away from the light, and this resulted in bending toward the light.
In each case, mutant plants that do not make the hormone exhibit a phenotype expected in the absence of the hormone, and adding the hormone reverses that phenotype (FIGURE 26.3):
- Tomato plants that do not make gibberellic acid are very short; supplying them with the hormone results in normal growth.
- Arabidopsis thaliana individuals that do not make auxin are also short; supplying them with that hormone reverses that phenotype.

Note that the phenotype involved—short stature, or dwarfism—is similar in both cases. This observation exemplifies a concept that is important to keep in mind when studying plant hormones: their actions are not unique and specific, as is the case with animal hormones (see Table 26.1).
560
The three-part approach outlined above for gibberellins and auxin—observation, hormone isolation, and analysis of mutant plants—has been used to identify other plant hormones.
Gibberellins have many effects on plant growth and development
The functions of gibberellins can be inferred from the effects of experimentally decreasing concentrations of gibberellins or blocking their action at various times in plant development. Such experiments reveal that gibberellins have multiple roles in regulating plant growth.
Stem Elongation
The effects of gibberellins on wild-type plants are not as dramatic as their effects on dwarf plants. We know, however, that gibberellins are indeed active in wild-type plants because inhibitors of gibberellin synthesis cause a reduction in stem elongation. Such inhibitors can be put to practical uses. For example, plants such as chrysanthemums that are grown in greenhouses tend to get tall and spindly. Because such plants do not appeal to consumers, flower growers spray them with gibberellin synthesis inhibitors to control their height.
Fruit Growth
Gibberellins can regulate the growth of fruit. Grapevines that produce seedless grapes develop smaller fruit than varieties that produce seed-bearing grapes. Biologists wanting to explain this phenomenon removed seeds from immature seeded grapes and found that this prevented normal fruit growth. This suggested that the seeds are sources of a growth regulator. Biochemical studies showed that developing seeds produce gibberellins, which diffuse out into the immature fruit tissue. Spraying young seedless grapes with a gibberellin solution, which causes them to grow as large as seeded ones, is now a standard commercial practice.

Mobilization of Seed Reserves
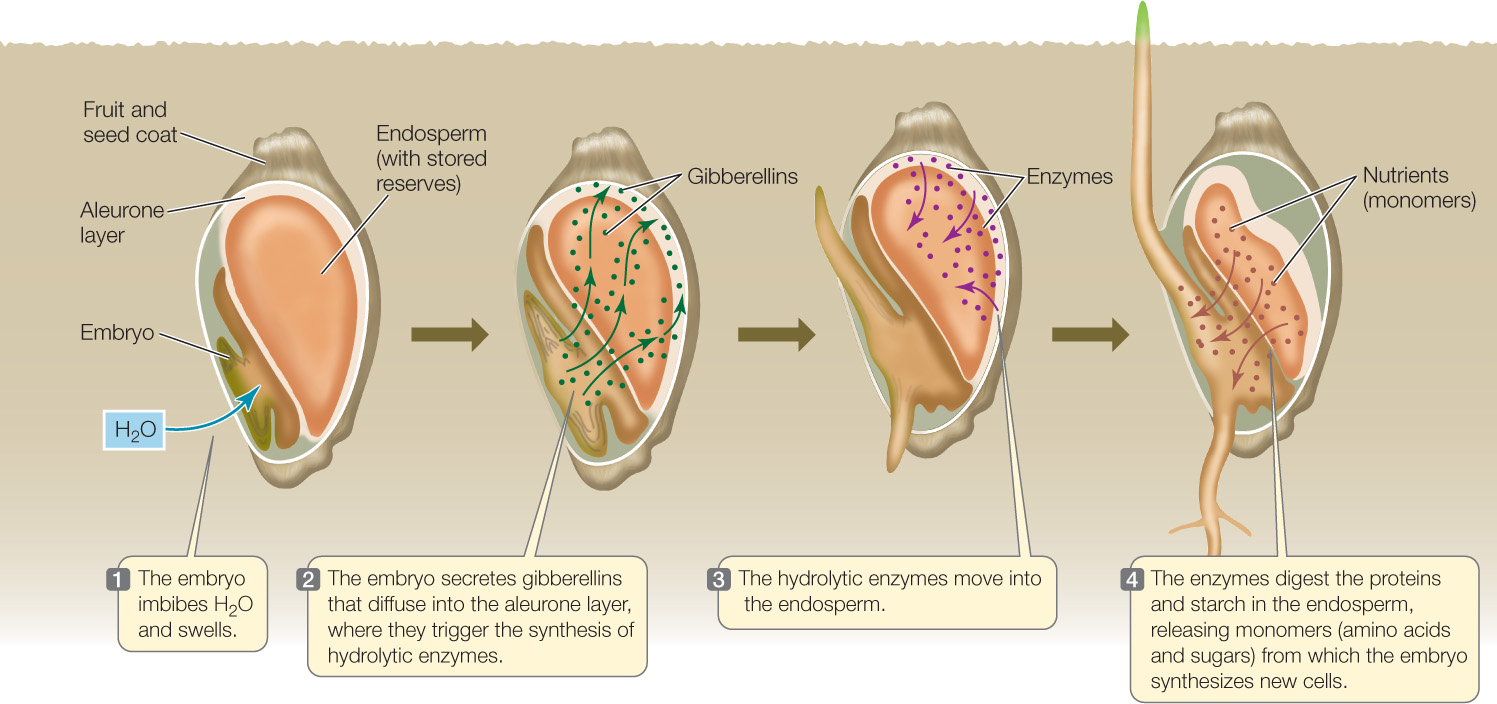
As noted in Concept 26.1, an important event early in seed germination is the hydrolysis of stored starch, proteins, and lipids. In germinating seeds of barley and other cereal crops, the embryo secretes gibberellins just after imbibition. The hormones diffuse through the endosperm to a surrounding tissue called the aleurone layer, which lies underneath the seed coat. The gibberellins trigger a cascade of events in the aleurone layer, causing it to synthesize and secrete enzymes that digest proteins and starch stored in the endosperm (FIGURE 26.4). These observations have practical importance: in the beer-brewing industry, gibberellins are used to enhance the “malting” (germination) of barley and the breakdown of its endosperm, producing sugars that are fermented into alcohol by yeast.
Go to ACTIVITY 26.3 Events of Seed Germination
PoL2e.com/ac26.3
561
The transport of auxin mediates some of its effects
As we noted above, auxin was discovered in the context of phototropism: a response to light in which plant stems bend toward a light source. Auxin is made in the shoot apex (tip) and diffuses down the shoot in a polar (unidirectional) fashion, stimulating cell expansion. Note that this use of “polar” differs from its use in chemistry, where it means hydrophilic (see Concept 2.2). Unidirectional longitudinal movement of auxin occurs in other plant organs as well. For example, in a leaf petiole, which connects the leaf blade to the stem, auxin moves from the leaf blade end toward the stem. In roots, auxin moves unidirectionally toward the root tip.
Polar transport of auxin depends on four biochemical processes that may be familiar from earlier chapters (FIGURE 26.5):
- Diffusion across a cell membrane. Polar (hydrophilic) molecules diffuse across cell membranes less readily than non-polar (hydrophobic) molecules (see Concept 5.2).
- Membrane protein asymmetry. Active transport, or efflux, carriers (see Concept 5.3) for auxin are located only in the portion of the cell membrane at the basal (bottom) end of the cell.
- Proton pumping/chemiosmosis. Proton pumps (see Concept 25.3) remove H+ from the cell, thereby increasing the pH inside the cell and decreasing the pH in the cell wall. Proton pumping also sets up an electrochemical gradient, which provides potential energy to drive the transport of auxin by the carriers mentioned above.
- Ionization of a weak acid. Indole-3-acetic acid (the chemical name for auxin; see Table 26.2) is a weak acid; in solution it forms ions (H+ and A−, where A− stands for indole acetate) that are in equilibrium with the non-ionized acid (HA):
A− + H+ ⇌ HA
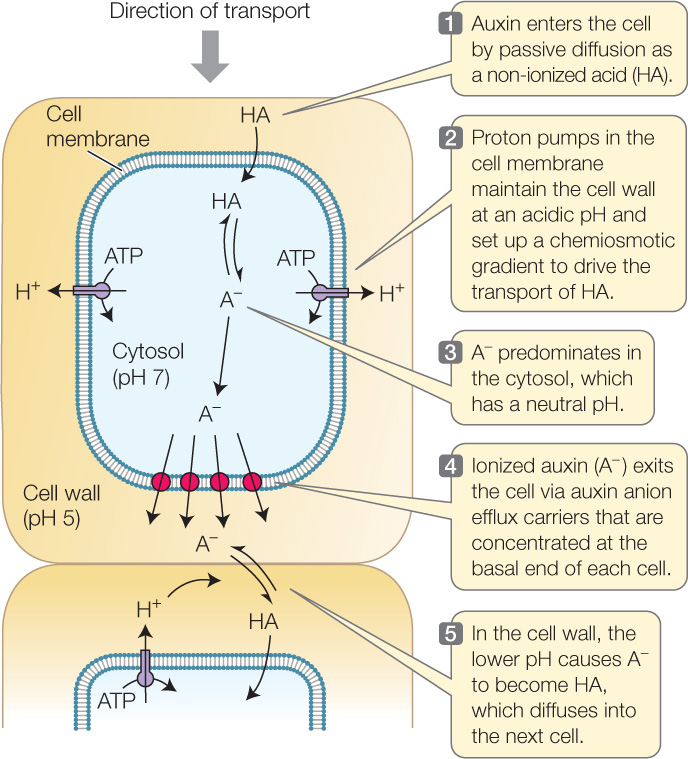
When the pH is low, the increased H+ concentration drives this reaction to the right, and HA (non-ionized auxin) is the predominant form. When the pH is higher, there is more A− (ionized auxin).
Whereas polar auxin transport distributes the hormone along the longitudinal axis of the plant, lateral (side-to-side) redistribution of auxin is responsible for directional plant growth. This redistribution is carried out by auxin efflux carriers that move from the base of the cell to one side; because of this, auxin exits the cell only on that side of the cell, rather than at the base, and moves sideways within the tissue.
APPLY THE CONCEPT: Gibberellins and auxin have diverse effects but a similar mechanism of action
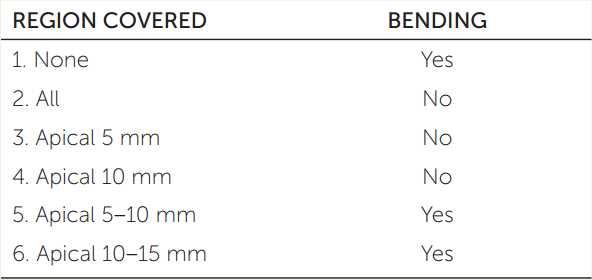
Oat seedlings with coleoptiles 20 mm long were exposed to light from one side and checked for bending after 6 hours (see photo on p. 562). Various regions of the seedlings were covered with foil to block the light. The results varied depending on which region of the coleoptile was covered (see table).
- Which part of the coleoptile senses the light?
- For each treatment, what would happen if supplementary auxin were applied to the coleoptiles on the same side as the light? On the side away from the light?
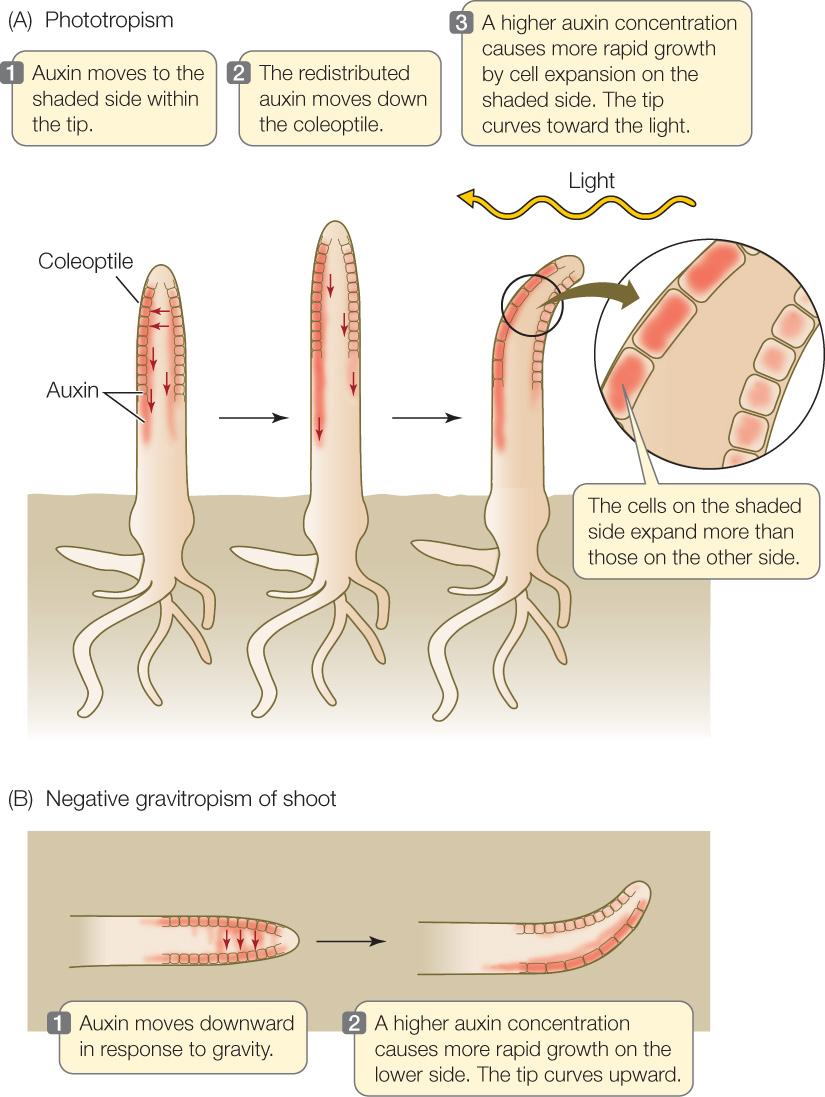
This lateral movement of auxin explains the bending of canary grass seedlings toward light. When light strikes a canary grass coleoptile on one side, auxin at the tip moves laterally toward the shaded side. The asymmetry thus established is maintained as polar transport moves auxin down the coleoptile, so that in the growing region below, the auxin concentration is highest on the shaded side. Cell expansion is thus increased on that side, causing the coleoptile to bend toward the light (FIGURE 26.6A). The same type of bending also occurs in eudicots:

Go to ANIMATED TUTORIAL 26.1 Tropisms
PoL2e.com/at26.1
562

Light is not the only signal that can cause redistribution of auxin. Auxin moves to the lower side of a shoot that has been tipped sideways, causing more rapid growth in the lower side and hence an upward bending of the shoot. Such growth in a direction determined by gravity is called gravitropism (FIGURE 26.6B). The upward gravitropic response of shoots is defined as negative gravitropism; that of roots, which bend downward, is positive gravitropism.
Auxin plays many roles in plant growth and development
Like the gibberellins, auxin has multiple roles in plant growth and development.
Root Initiation
Cuttings from the shoots of some plants can produce roots and develop into entire new plants. For this to occur, cells in the interior of the shoot, originally destined to function only in food storage, must change their fate and become organized into the apical meristem of a new root (this is an example of the remarkable totipotency of plant cells; see Concept 14.1). Shoot cuttings of many species can be made to develop roots by dipping the cut surfaces into an auxin solution. These observations suggest that in an intact plant, the plant’s own auxin plays a role in the initiation of lateral roots.
Leaf Abscission
In contrast to its stimulatory effect on root initiation, in leaves auxin inhibits the detachment of old leaves from stems. This detachment process, called abscission, is the cause of autumn leaf fall. Recall that the blade of a leaf produces auxin that moves toward the petiole. If the blade is cut off, the petiole falls from the plant more rapidly than if the leaf had remained intact. If the cut surface is treated with an auxin solution, however, the petiole remains attached to the plant, often longer than an intact leaf would have. The timing of leaf abscission in nature appears to be determined in part by a decrease in the movement of auxin through the petiole.
Apical Dominance
Auxin helps maintain apical dominance, a phenomenon in which the apical bud (the very top/first bud that will form leaves) inhibits the growth of axillary buds (side buds; see Figure 24.1), resulting in the growth of a single main stem with minimal branching. A diffusion gradient of auxin from the apex of the shoot down the stem results in lower branches receiving less auxin and therefore branching more, while higher branches receive more auxin and branch less.
Fruit Development
Fruit development normally depends on prior fertilization of the ovule (egg), but in many species, treatment of an unfertilized ovary with auxin or gibberellins causes parthenocarpy: fruit formation without fertilization. Parthenocarpic fruits form spontaneously in some cultivated varieties of plants, including seedless grapes, bananas, and some cucumbers.
563
Cell Expansion
The expansion of plant cells is a key process in plant growth. Because the plant cell wall normally resists expansion of the cell, it plays a key role in controlling the rate and direction of plant cell growth. Auxin acts on the cell wall to regulate this process.
The expansion of a plant cell is driven primarily by the uptake of water, which enters the cell by osmosis. As it expands, the cell contents press against the cell wall, which resists this force (producing turgor pressure; see Figure 25.8). The cell wall is an extensively cross-linked network of polysaccharides and proteins, dominated by cellulose fibrils (see Figures 2.10 and 4.15). If the cell is to expand, some adjustments must be made in the wall structure to allow the wall to “give” under turgor pressure. Think of a balloon (the cell surrounded by a membrane) inside a box (the cell wall).
LINK
Water movement and turgor pressure in plant cells are discussed in Concept 25.3
The acid growth hypothesis explains auxin-induced cell expansion (FIGURE 26.7). This hypothesis proposes that protons (H+) are pumped from the cytoplasm into the cell wall, lowering the pH of the wall and activating enzymes called expansins, which catalyze changes in the cell wall structure such that the polysaccharides adhere to each other less strongly. This change loosens the cell wall, allowing it to stretch as the cell expands. Auxin is believed to have two roles in this process: to increase the synthesis of proton pumps, and to guide their insertion into the cell membrane. Several lines of evidence support the acid growth hypothesis. For example, adding acid to the cell wall to lower the pH stimulates cell expansion even in the absence of auxin. Conversely, when a buffer is used to prevent the wall from becoming more acidic, auxin-induced cell expansion is blocked.
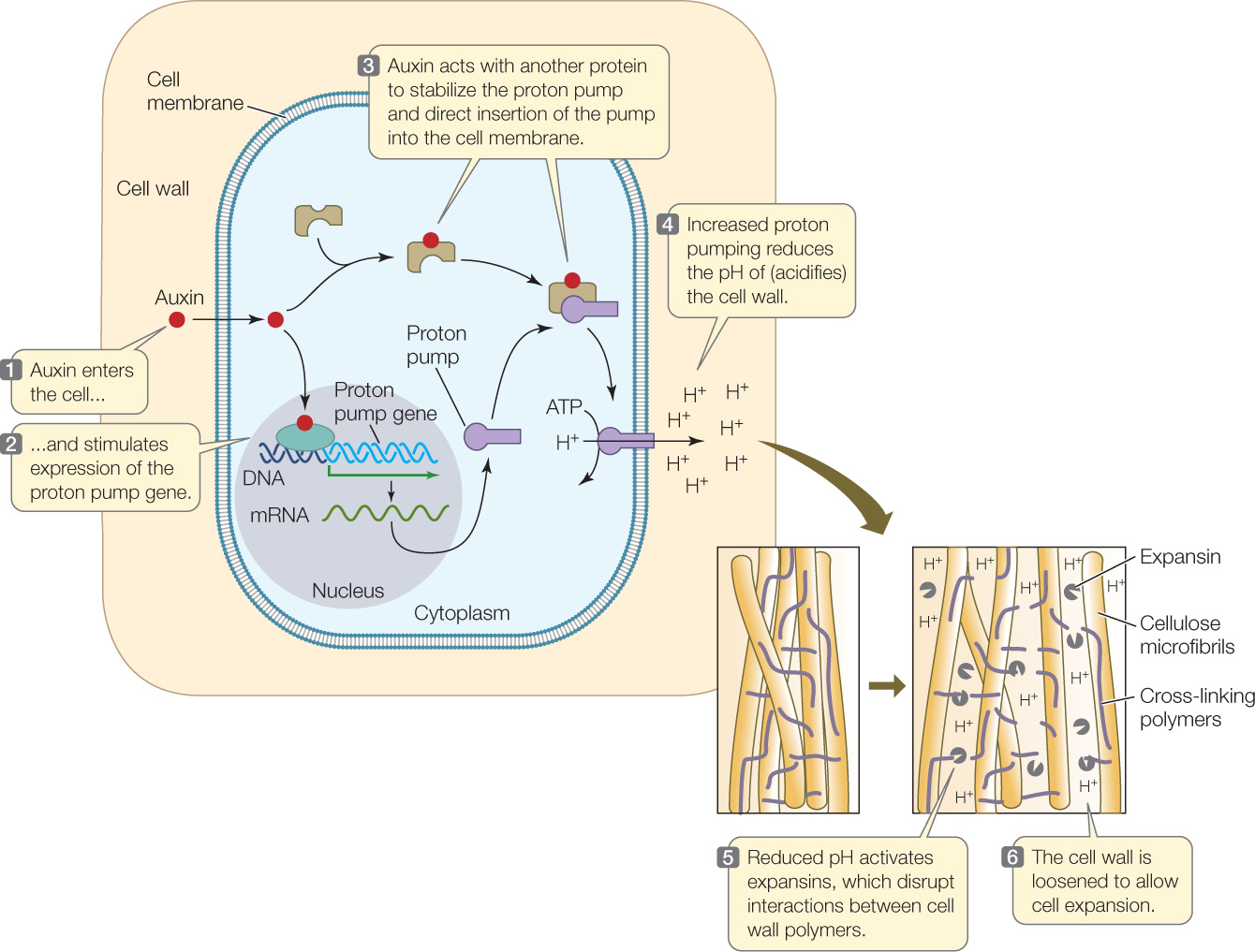

Go to ANIMATED TUTORIAL 26.2 Auxin Affects Cell Walls
PoL2e.com/at26.2
At the molecular level, auxin and gibberellins act similarly
The molecular mechanisms underlying both auxin and gibberellin action have been worked out with the help of genetic screens (see Figure 26.2). Biologists started by identifying mutant plants whose growth and development are insensitive to the hormones; that is, plants that are not affected by added hormone. Such mutant plants fall into two general categories:
- Excessively tall plants. These plants resemble wild-type plants given an excess of hormone, and they grow no taller when given extra hormone. They grow tall even when treated with inhibitors of hormone synthesis. Their hormone response is always “on,” even in the absence of the hormone. It is presumed that the normal allele for the mutated gene codes for an inhibitor of the hormone signal transduction pathway. In wild-type plants, the signal transduction pathway is “off” unless stimulated by the hormone—therefore the plants are sensitive to added hormone. In the mutant plants the pathway is always “on”; the plant grows tall and is insensitive to added hormone.
- Dwarf plants. These plants resemble dwarf plants that are deficient in hormone synthesis (see Figure 26.3), but they do not respond to added hormone. In these mutant plants the hormone response is always “off,” regardless of the presence of the hormone.
564
APPLY THE CONCEPT: Gibberellins and auxin have diverse e3 ects but a similar mechanism of action
The aleurone layer of germinating barley can be isolated and studied for the induction of α-amylase, the enzyme that catalyzes starch hydrolysis. Predict the amount of α-amylase activity in aleurone layers subjected to the following treatments:
- Incubation with gibberellic acid
- Incubation with auxin
- Incubation with gibberellic acid in the presence of an inhibitor of DNA transcription to mRNA
- Incubation without gibberellic acid
- Incubation without gibberellic acid in the presence of an inhibitor of transcription
- Incubation with gibberellic acid in the presence of an inhibitor of the proteasome
- Incubation without gibberellic acid in the presence of an inhibitor of the proteasome
Remarkably, some mutations of both types turned out to affect the same gene. How could this be? The mystery was solved once biologists figured out how auxin and gibberellins work in wild-type plants. Of course, the actual proteins involved with the two hormones are different, but the actions of both hormones are similar: they act by removing a repressor from a transcription factor that stimulates the expression of growth-promoting genes (FIGURE 26.8). The hormones bind to a receptor protein, which in turn binds to the repressor. Binding of the hormone–receptor complex stimulates polyubiquitination of the repressor, targeting it for breakdown in the proteasome (see Figure 11.19).
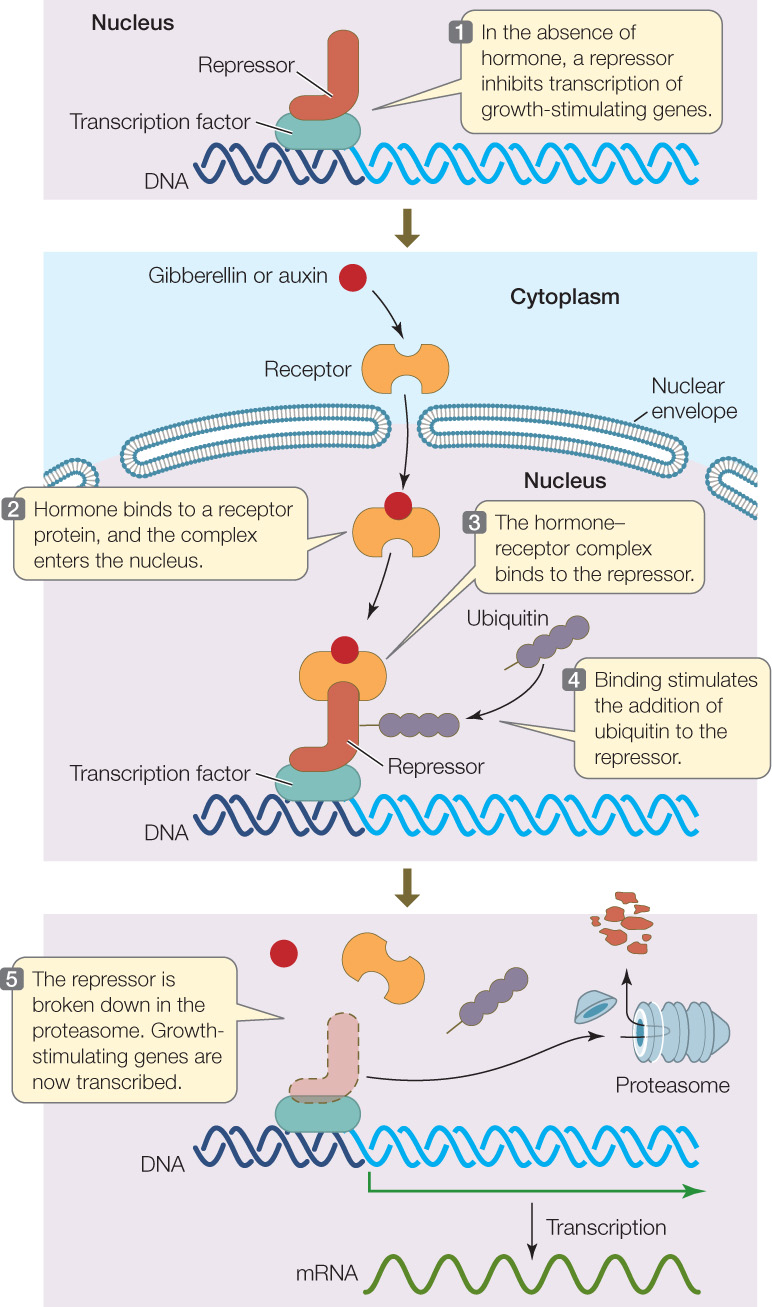

Go to MEDIA CLIP 26.1 Gibberellin Binding to Its Receptor
PoL2e.com/mc26.1
LINK
Review the process of transcription initiation in Concept 11.1
The features of this pathway explain how different mutations in the same gene can have seemingly opposite phenotypes. The mutations described above turned out to be in the gene that encodes the repressor protein. One region of the repressor protein binds to the transcription factor. This is the mutated region in the excessively tall plants: the growth-promoting genes are always “on” because the repressor does not bind to the transcription factor to inhibit transcription. Another region of the repressor protein causes it to be removed from the transcription factor. This is the mutated region in the dwarf plants: the growth-promoting genes are always “off” because the repressor is always bound to the transcription factor.
The receptors for both auxin and gibberellins contain a region called an F-box that facilitates protein–protein interactions necessary for protein breakdown. Whereas animal genomes have few F-box–containing proteins, plant genomes have hundreds, an indication that this type of gene regulation is common in plants.
565
CHECKpoint CONCEPT 26.2
- Make a table for one auxin response and one gibberellin response with three column headings: site of synthesis, site of action, and effect.
- Explain why, even though auxin moves away from the lighted side of a coleoptile tip, the coleoptile bends toward the light.
- What would be the phenotype, in terms of seed germination, of a plant that has a mutation that disables the F-box region of the receptor for gibberellin?
The same approaches that led to the discovery of auxin and gibberellins have revealed several other classes of plant hormones. These hormones are diverse in their actions and affect many of the same developmental processes as auxin and gibberellins.