Concept 27.2: Hormones and Signaling Determine the Transition from the Vegetative to the Reproductive State
Flowering represents a reallocation of energy and materials away from making more roots, stems, and leaves (vegetative growth) to making flowers and gametes (reproductive growth). Flowering can happen at maturity as part of a predetermined developmental program (as in a dandelion plant in summer) or in response to environmental cues such as light or temperature (as with the poinsettias we described at the opening of this chapter). In either case, flowering is initiated through a cascade of changes in gene expression.
Herbaceous plants fall into three categories based on when they mature and initiate flowering and what happens after they flower:
- Annuals complete their lives within a year. Annuals include many crops important to humans, such as corn, wheat, rice, and soybeans. When the environment is suitable, annuals grow rapidly. After flowering, they channel most of their energy into the development of seeds and fruits. Then the rest of the plant withers and dies.
- Biennials take two years to complete their life cycle. They are much less common than annuals and include carrots, cabbage, and onions. Typically, biennials produce only vegetative growth during their first year and store carbohydrates in underground roots (carrots) or stems (onions). In their second year, they use most of the stored carbohydrates to produce flowers and seeds rather than vegetative growth, and the plant dies after seeds form.
- Perennials live three or more—sometimes many more—years. Maple trees, for example, can live up to 400 years. Perennials include many wildflowers as well as trees and shrubs. Typically these plants flower each year but stay alive and keep growing the next season.
Shoot apical meristems can become inflorescence meristems
The transition from purely vegetative growth to the flowering state requires a change in one or more apical meristems in the shoot system. As we described in Chapter 24, shoot apical meristems continually produce leaves, axillary buds, and stem tissues (FIGURE 27.6A) in a kind of unrestricted growth called indeterminate growth.

A shoot apical meristem becomes an inflorescence meristem (recall that an inflorescence is a cluster of flowers; see Figure 21.19) when it ceases production of leaves and stems and produces other structures: smaller leafy structures called bracts (such as the red leaves in poinsettias), as well as new meristems in the angles between the bracts and the stem (FIGURE 27.6B). These new meristems may also be inflorescence meristems, or they may be floral meristems, each of which gives rise to a single flower.
580
Each floral meristem typically produces four consecutive whorls, or spirals, of organs—the sepals, petals, stamens, and carpels—separated by very short internodes, which keep the flower compact (FIGURE 27.6C). In contrast to shoot apical meristems and some inflorescence meristems, floral meristems are responsible for determinate growth—growth of limited extent, like that of leaves.
A cascade of gene expression leads to flowering
The genes that determine the transition from shoot apical meristems to inflorescence meristems and from inflorescence meristems to floral meristems have been studied in model organisms such as Arabidopsis thaliana.
Meristem Identity Genes
Expression of two meristem identity genes initiates a cascade of further gene expression that leads to flower formation. Expression of the genes LEAFY and APETALA1 is both necessary and sufficient for flowering. Evidence for the role of these genes is both genetic and molecular. For example, a mutant allele of APETALA1 leads to continued vegetative growth, even if conditions are suitable for flowering. However, if plants are genetically modified so that the wild-type APETALA1 gene is strongly expressed in the shoot apical meristem, the plant will flower regardless of environmental conditions. This is powerful evidence that APETALA1 plays a role in switching shoot apical meristem cells from a vegetative to a reproductive fate (see Figure 27.6B).
Floral Organ Identity Genes
The products of the meristem identity genes trigger the expression of floral organ identity genes, which work in concert to specify the successive whorls of the flower (see Figure 27.6C). Floral organ identity genes are homeotic genes that cause the development of specific structures. In plants, the products of homeotic genes are transcription factors that determine whether cells in the floral meristem will be sepals, petals, stamens, or carpels. An example is the gene AGAMOUS, a class C gene that causes florally determined cells to form stamens and carpels in the “ABC” system described in Chapter 14 (see Figure 14.12).
Depending on the species, plants initiate these gene expression changes, and the events that follow, in response to either internal or external cues. Among external cues, the most studied are photoperiod (day length) and temperature. We will begin with photoperiod.
Photoperiodic cues can initiate flowering
The study of how light affects the transition to flowering began with two observations in the early twentieth century:
- A mutant tobacco strain appropriately called Maryland Mammoth grew 5 meters tall (normal tobacco is about 1.5 m tall). Instead of flowering in summer, it continued to grow until the late fall frost killed it. Farmers in Virginia were frustrated because they could not get seed of this luxuriant plant for the next year’s crop.

- Because of improvements in agricultural techniques, soybean yields became so great that it was hard for farmers to harvest all the plants at once. So they tried planting the seeds in groups, several weeks apart. Unfortunately, the resulting plants all formed flowers and seeds at the same time.
The explanation for both of these observations was the same: the signal that set the plants’ shoot apical meristems on the path to flowering was the length of daylight, or photoperiod. When soybeans experienced days of a certain length, they flowered, regardless of how “old” they were. Maryland Mammoth tobacco could flower, but it did not do so in Virginia because it died when the weather got cold. When the plant was grown in a greenhouse to prevent freezing, however, it flowered in December, when the days were short. Maryland Mammoth is now grown commercially in Florida.
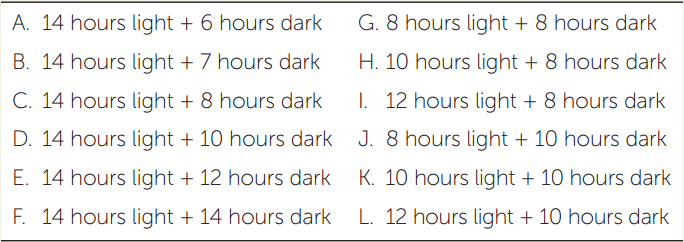
Greenhouse experiments measured the day length required for different plant species to flower. Maryland Mammoth tobacco did not flower if exposed to more than 14 hours of light per day; flowering was only initiated once day length became shorter than 14 hours, as it does in December. Other plants (such as soybeans and henbane) flowered only when the days were long (FIGURE 27.7).

Plants vary in their responses to photoperiodic cues
Plants that flower in response to photoperiodic stimuli fall into two main classes, although there are variations on these patterns:
- Short-day plants (SDPs) flower only when the day is shorter than a critical maximum. They include poinsettias and chrysanthemums as well as Maryland Mammoth tobacco. Thus, for example, we see chrysanthemums in nurseries in fall and poinsettias in winter (as noted at the opening of this chapter).
- Long-day plants (LDPs) flower only when the day is longer than a critical minimum. Spinach and clover are examples of LDPs. Spinach tends to flower and become bitter in the summer and is therefore normally planted in early spring.
Photoperiodic control of flowering serves an important role: it synchronizes the flowering of plants of the same species in a local population. This synchronization promotes cross-pollination and successful reproduction. It also means that floriculturists can vary light exposures in greenhouses to produce flowers at any time of year.
Night length is the key photoperiodic cue that determines flowering
The terms “short-day plant” and “long-day plant” assume that the key events that induce flowering occur in the daytime. What about the night? After all, short-day plants could just as easily be called “long-night plants,” and long-day plants could be called “short-night plants.” As it turns out, night length is indeed the critical factor that induces flowering, as a series of greenhouse experiments confirmed (FIGURE 27.8). In a greenhouse, the overall length of a day or night can be varied irrespective of the 24-hour natural cycle. For example, if cocklebur, an SDP, is exposed to several long periods of light (16 hours each), it will still flower as long as the dark period between them is 9 hours or longer. This 9-hour inductive dark period also induces flowering even if the light period varies from 8 hours to 12 hours.

Biologists noticed that when the inductive dark period was interrupted by a brief period of light, the flowering signal generated by the long night disappeared. It took several days of long nights for the plant to recover and initiate flowering. Interrupting the day with a dark period had no effect on flowering. A clue as to what occurred in the plant when the flash of light was given came when biologists determined the action spectrum for the wavelengths of light that were effective. As with lettuce seed germination (see Figure 26.11), red light was most effective at breaking the “night” stimulus, and its effect was reversible by far-red light. This indicated that the photoreceptor involved in regulating flowering is phytochrome.
LINK
The properties of phytochrome as a photoreversible photoreceptor of red and far-red light are described in Concept 26.4

Go to ANIMATED TUTORIAL 27.2 The Effect of Interrupted Days and Nights
PoL2e.com/at27.2
Plants sense night length by measuring the ratio of the Pfr and Pr isoforms of phytochrome. During the day there is more light in the red than in the far-red range. By the end of the day, much of the Pr has absorbed red light and been converted to Pfr. At night there is a gradual, spontaneous conversion of this Pfr back to Pr. The longer the night, the more Pr there is at the beginning of the next day. An SDP flowers when the ratio of Pfr to Pr is low at the end of the night, whereas an LDP flowers when this ratio is high. You can compare this mechanism to an hourglass egg timer. The sand in the top chamber is analogous to Pfr and gradually runs down (is converted to Pr) over the course of the night; the shorter the night, the more sand remains at the top at dawn. To “reset” an hourglass timer, you simply turn it over; this would be analogous to dawn of the next day, when Pr begins being converted back to Pfr.
582
APPLY THE CONCEPT: Hormones and signaling determine the transition from the vegetative to the flowering state
The experiments reported on in Figure 27.8 were repeated using a long-day plant (LDP) that normally flowers when days are 16 hours (or longer) and nights are 8 hours (or shorter). The plant was subjected to the light regimes listed in the table.
- List the light regimes that should have resulted in flowering.
- Predict the effect of adding a brief period of far-red light in the middle of the dark period of each light regime.
- Predict the effect of adding a brief period of red light in the middle of the dark period of each light regime.
The flowering stimulus originates in the leaf
The photoperiodic receptor for flowering, phytochrome, is located in the leaf, not in the shoot apical meristem. “Masking” experiments can show this: if a single leaf of an SDP growing under short-night conditions is covered to simulate a long-night exposure, the shoot apical meristems will transition to flowering as if the entire plant were exposed to long nights (FIGURE 27.9). Masking the buds (leaving the leaves exposed) does not induce flowering.
Investigation
HYPOTHESIS
The leaves measure the photoperiod.
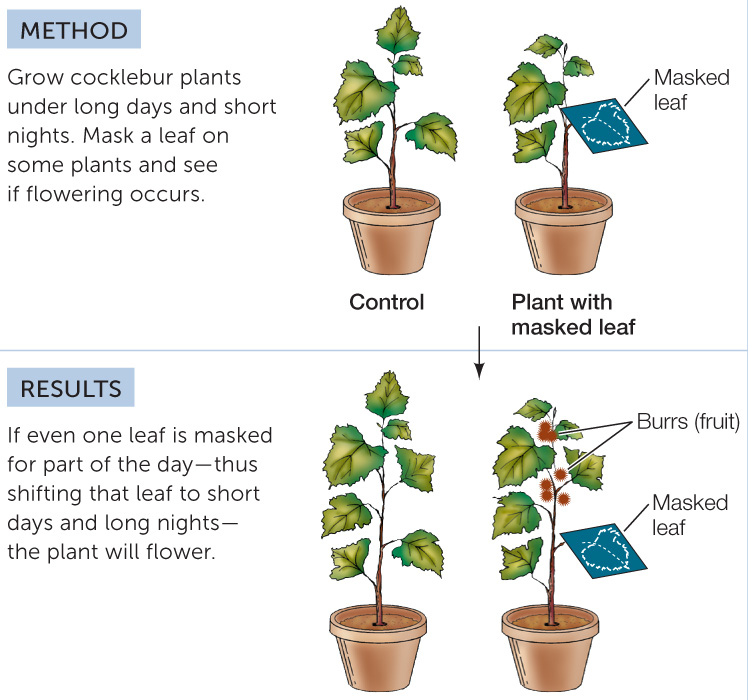
CONCLUSION
The leaves measure the photoperiod. Therefore, some signal must move from the induced leaf to the flowering parts of the plant.
ANALYZE THE DATA
In related experiments, leaves were removed from plants before the plants were exposed to the inductive dark period. There were six plants in each condition.b

- Do the data support the conclusion of the experiment by Knott described above? Explain your answer.
- How would you modify the experiments shown in the table to find out how many days of the inductive dark period are required before the signal is produced? Would you expect the results to be different for the intact plants and plants with only one leaf? Why or why not?
Go to LaunchPad for discussion and relevant links for all INVESTIGATION figures.
aJ. E. Knott. 1934. Proceedings of the Society of Horticultural Science 31: 152–154.
bK. C. Hamner and J. Bonner. 1938. Botanical Gazette 100: 388–431.
Because the receptor of the photoperiodic stimulus (phytochrome in the leaf) is physically separated from the tissue on which the stimulus acts (the shoot apical meristem), the inference can be drawn that a signal travels from the leaf through the plant’s tissues to the shoot apical meristem. Unlike animals, plants do not have a nervous system, so the signal must be a diffusible chemical. Biologists have found additional evidence that a diffusible signal travels from the leaf to the shoot apical meristem:
- If a photoperiodically induced leaf is immediately removed from a plant after the inductive dark period, the plant does not flower. If the induced leaf remains attached to the plant for several hours, however, the plant flowers. This result suggests that something is synthesized in the leaf in response to the inductive dark period, and then moves out of the leaf to induce flowering.
- If two or more cocklebur plants are grafted together, and if one plant is exposed to inductive long nights and its graft partners are exposed to noninductive short nights, all of the plants flower.
- In several species, if an induced leaf from one species is grafted onto another, noninduced plant of a different species, the recipient plant flowers. This indicates that the same diffusible chemical signal is used by both species.
Although the diffusible signal was given a name, florigen (FT), meaning “flower inducing,” decades ago, the nature of the signal was only recently explained.
Florigen is a small protein
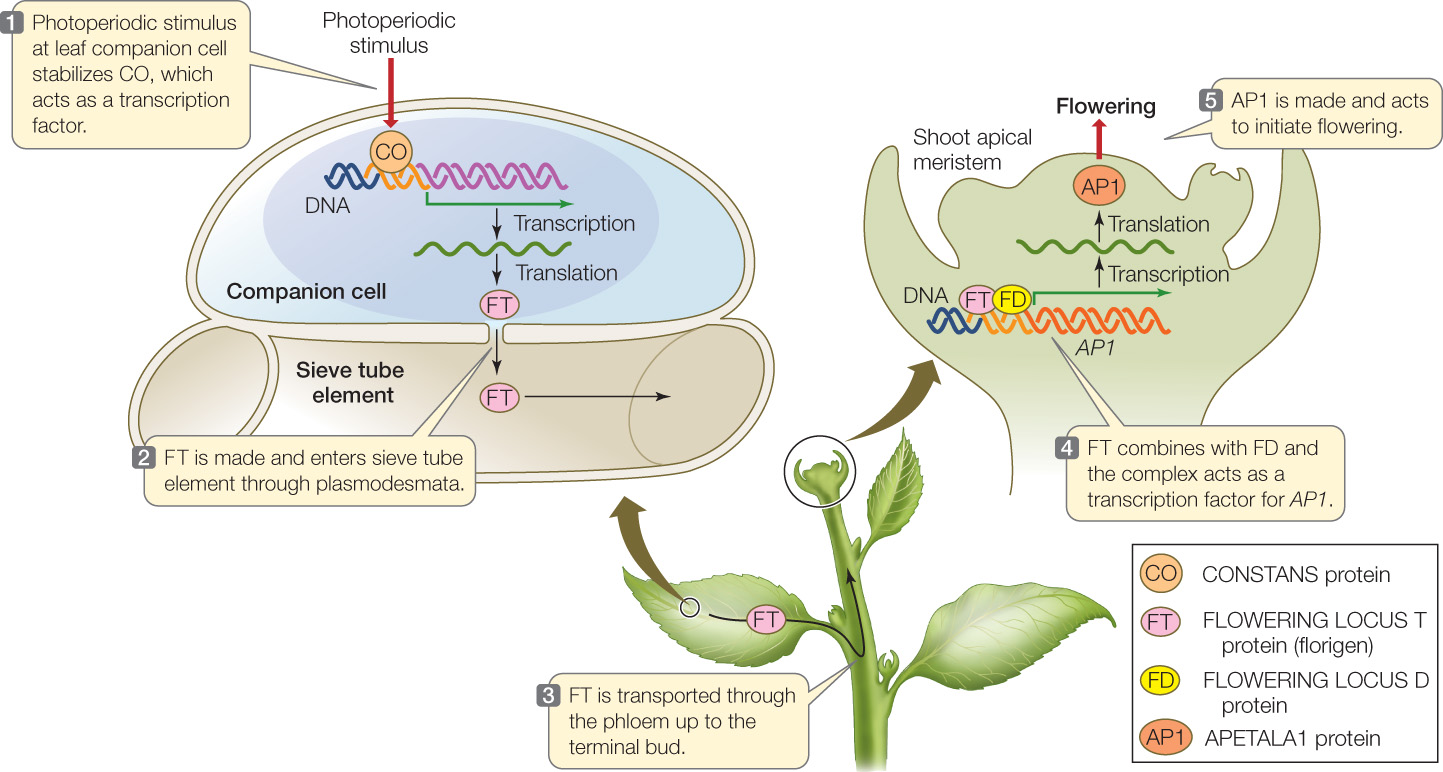
The characterization of florigen was made possible by genetic and molecular studies of the model organism Arabidopsis, an LDP. Three genes are involved (FIGURE 27.10):
- FT (FLOWERING LOCUS T) codes for florigen. FT is a small protein (20 kDa molecular weight) that can travel through plasmodesmata. It is synthesized in phloem companion cells in the leaf and diffuses into the adjacent sieve tube elements, where it moves with the phloem to the shoot apical meristem.
- CO (CONSTANS) codes for a transcription factor that activates the synthesis of FT. Like FT, CO is expressed in phloem companion cells in the leaf. CO protein is expressed all the time but is unstable; an appropriate photoperiodic stimulus stabilizes CO so that there is enough to turn on FT synthesis.
- FD (FLOWERING LOCUS D) codes for a protein that binds to FT protein when it arrives in the shoot apical meristem. The FD protein is a transcription factor that forms a complex with FT protein; the complex activates promoters for meristem identity genes, such as APETALA1 (AP1).
Genetic studies have shown that the FT gene is involved in photoperiod signaling in many species:
- Transgenic plants (e.g., tobacco and tomato) that express the Arabidopsis FT gene at high levels flower regardless of day length.
- Transgenic Arabidopsis plants that express high levels of FT genes from other plants (e.g., rice and tomato) flower regardless of day length.
Whereas photoperiod induces flowering via the FT–FD pathway in some plants, in many other species flowering is induced by other stimuli through other pathways.
583
584
Flowering can be induced by temperature or gibberellins
Temperature
In some plant species, notably certain cereal crops, the environmental signal for flowering is exposure to cold temperatures, or vernalization (Latin vernus, “spring”). In both wheat and rye, we can distinguish two categories of flowering behavior. Spring wheat, for example, is a typical annual plant: it is sown in the spring and flowers in the same year. Winter wheat is sown in the fall, grows into a seedling, overwinters (often covered by snow), and flowers the following summer. If winter wheat is not exposed to cold, it will not flower normally in the next growing season. Vernalization evolved in plants adapted to grow in climates with harsh winters and a short growing season. It is a remarkable phenomenon:
- Plants seem to “remember” that they have been exposed to winter. When the weather gets warmer, exposed plants flower sooner than genetically identical plants that were not exposed to cold.
- Plants seem to “know” how long winter lasted, flowering sooner if the winter was long.
Some strains of Arabidopsis require vernalization, and those strains have been useful in figuring out the molecular pathway involved (FIGURE 27.11). The gene FLC (FLOWERING LOCUS C) encodes a transcription factor that blocks the FT–FD pathway by inhibiting expression of FT and FD. Cold temperatures inhibit the synthesis of FLC protein epigenetically: the chromatin is modified so that the FLC gene is not expressed (it is silenced). The longer the cold, the more cells there are that have the FLC gene silenced. This allows the FT and FD proteins to be expressed and flowering to proceed. Similar proteins control some steps in vernalization in cereals.

LINK
Chromatin modification is a common way to regulate gene expression; see Concept 11.3
In addition to the well-known effects of cold temperature, there is also an effect of elevated temperature on flowering. Recently, biologists identified a transcription factor that increases the expression of the florigen gene FT and is more active at elevated temperatures (27°C as opposed to 22°C). The plants exposed to warm temperatures flower earlier, typically with smaller flowers and fewer seeds. This has important implications for the effects of climate change on crops. As Earth’s climate gets warmer, food production may be reduced because of premature activation of the flowering pathway.
Gibberellins
Arabidopsis plants do not flower if they are genetically deficient in gibberellin synthesis or if they are treated with an inhibitor of gibberellin synthesis. These observations implicate gibberellins in flowering. Direct application of gibberellins to buds in Arabidopsis results in activation of the meristem identity gene LEAFY, which in turn promotes the transition to flowering (see Concept 14.3).
Some plants do not require an environmental cue to flower
Some plants flower in response to cues from an “internal clock.” For example, flowering in some strains of tobacco is initiated in the terminal bud when the stem has grown four phytomers in length (recall that stems are composed of repeating units called phytomers; see Figure 24.1). If a terminal bud and a single adjacent phytomer are removed from a plant this size and planted, the cutting will flower because the bud has already received the cue for flowering. But the rest of the shoot below the bud that has been removed will not flower because it is only three phytomers long. After it grows an additional phytomer, it will flower. These results suggest that there is something about the position of the bud (atop four phytomers of stem) that determines its transition to flowering.
LINK
The role of positional information in morphogenesis in both plants and animals is described in Concept 14.3
The bud might “know” its position by the concentration of some substance that forms a positional gradient along the apical–basal axis of the plant. Such a gradient could be formed, for example, if the root makes a diffusible inhibitor of flowering whose concentration diminishes with plant height. When the plant reached a certain height, the concentration of the inhibitor would become sufficiently low at the tip of the shoot to allow flowering. The relationship between environmental factors and flowering are summarized below:

585
APPLY THE CONCEPT: Hormones and signaling determine the transition from the vegetative to the flowering state
Describe the genes and mutations that could be involved in each of the following observations:
- A mutant plant flowers without its normal inductive dark period. When a leaf from the mutant plant is grafted onto an unexposed wild-type plant, the recipient plant flowers.
- A mutant plant does not flower when exposed to the normal inductive dark period. When a leaf from a mutant plant that has been exposed to the inductive dark period is grafted onto an unexposed wild-type plant, the recipient plant flowers.
- A plant that does not usually require vernalization flowers only after exposure to cold.
- If a gene is coupled to an active promoter and expressed at high levels in the shoot apical meristem, flowering is induced even in the absence of an appropriate photoperiodic stimulus.
- If a gene is experimentally overexpressed in the leaf, flowering is induced. Overexpression of the gene in the shoot apical meristem does not, however, induce flowering.
CHECKpoint CONCEPT 27.2
- How do shoot apical meristems, inflorescence meristems, and floral meristems differ, and what are the genes that control the transitions between them?
- Explain why “short-day plant” is a misleading term.
- If an LDP is exposed to its inductive dark period and one of its leaves is removed and grafted onto an SDP that has not been exposed to its inductive dark period, what will happen?
- How would you show that vernalization acts through the FLC gene?
Sexual reproduction is not the only way in which angiosperms give rise to more plants. We will now turn to some ways in which plants can reproduce asexually.