Concept 28.2: Plants Have Mechanical and Chemical Defenses against Herbivores
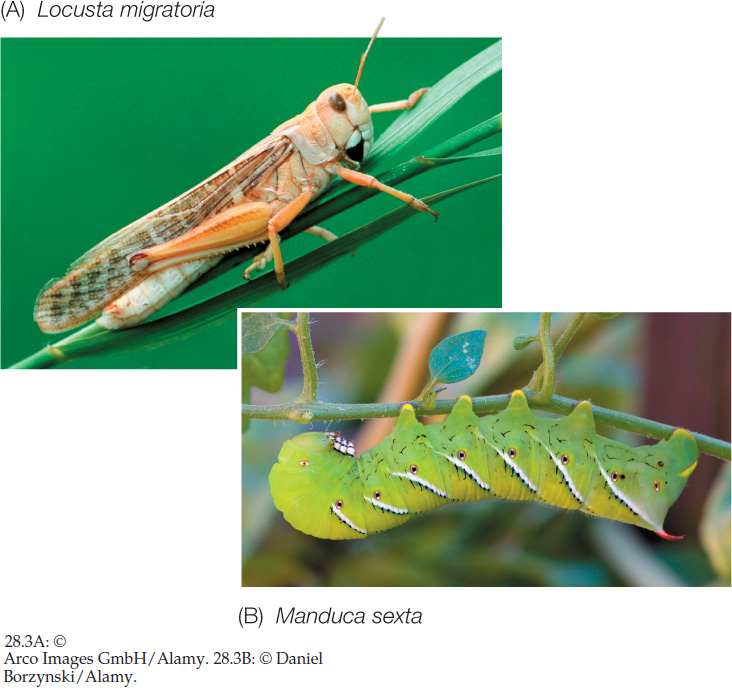
Herbivores depend on plants for energy and nutrients. Their foraging activities cause physical damage to plants, and they often spread pathogens among plants as well. While the majority of herbivores are insects (FIGURE 28.3), most other groups of animals also include some herbivores. Plants have both constitutive and induced mechanisms to protect themselves from herbivory.
Constitutive defenses are physical and chemical
Plants have a number of constitutive anatomical features, such as trichomes (leaf hairs), thorns, and spines that may be specialized for defense. Some plants have insoluble crystals of salts such as calcium oxalate that damage the tissues of insects that consume the plants. In addition, normal plant features, including thick cell walls and tree bark, may deter some herbivores.
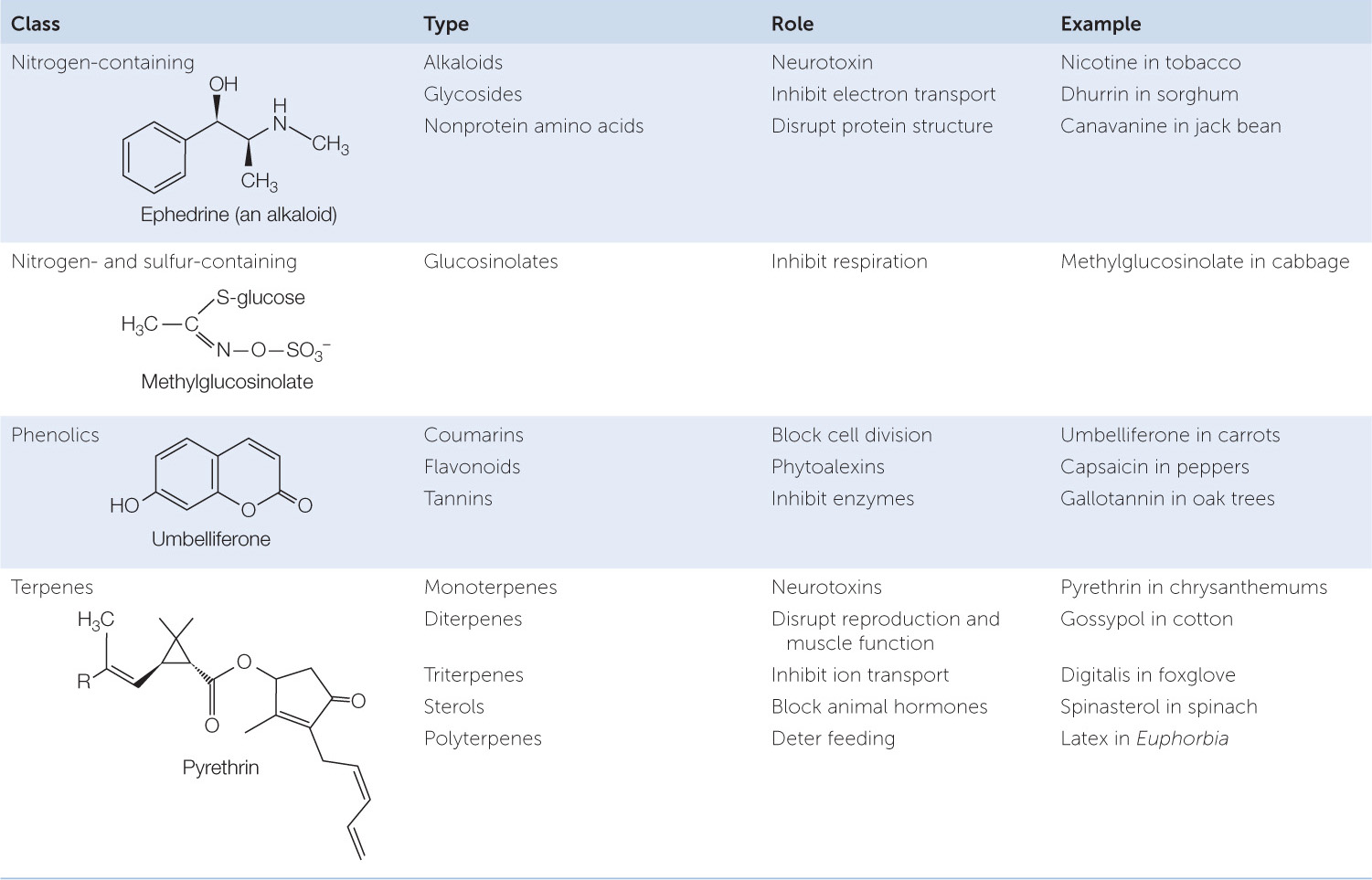
Plants resort to “chemical warfare” to repel or inhibit other herbivores, using special chemicals called secondary metabolites. These substances are referred to as “secondary” because, unlike molecules such as sugars and amino acids, they are not used for basic cellular processes. The more than 10,000 known secondary metabolites range in molecular mass from about 70 to more than 390,000 daltons, but most are small (TABLE 28.1). Some are produced by only a single plant species, whereas others are characteristic of entire genera or even families. The effects of defensive secondary metabolites on animals are diverse. Some act on the nervous systems of herbivorous insects, mollusks, or mammals. Others mimic the natural hormones of insects, causing some larvae to fail to develop into adults. Still others damage the digestive tracts of herbivores.
An example of a secondary metabolite is canavanine, an amino acid that is similar to the amino acid arginine:
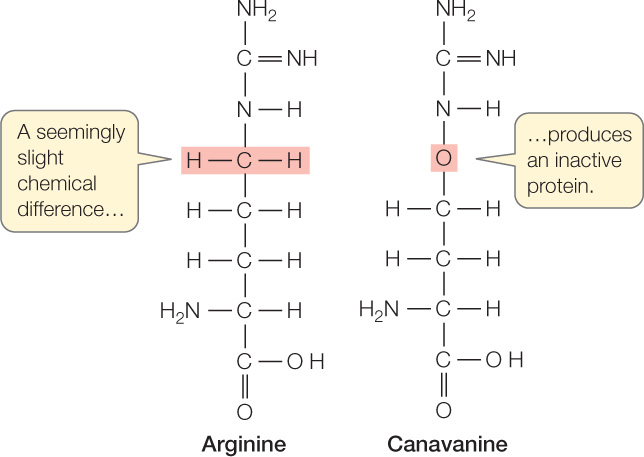
When an insect larva consumes canavanine-containing plant tissue, the canavanine is incorporated into the insect’s proteins in some of the places where the insect’s mRNA codes for arginine. The enzyme that charges the tRNA specific for arginine fails to discriminate accurately between the two amino acids. The structure of canavanine, however, is different enough from that of arginine that some of the resulting proteins end up with a modified tertiary structure and hence reduced biological activity. These defects in protein structure and function lead to developmental abnormalities that kill the insect.
594
LINK
The charging of tRNA is described in Concept 10.4
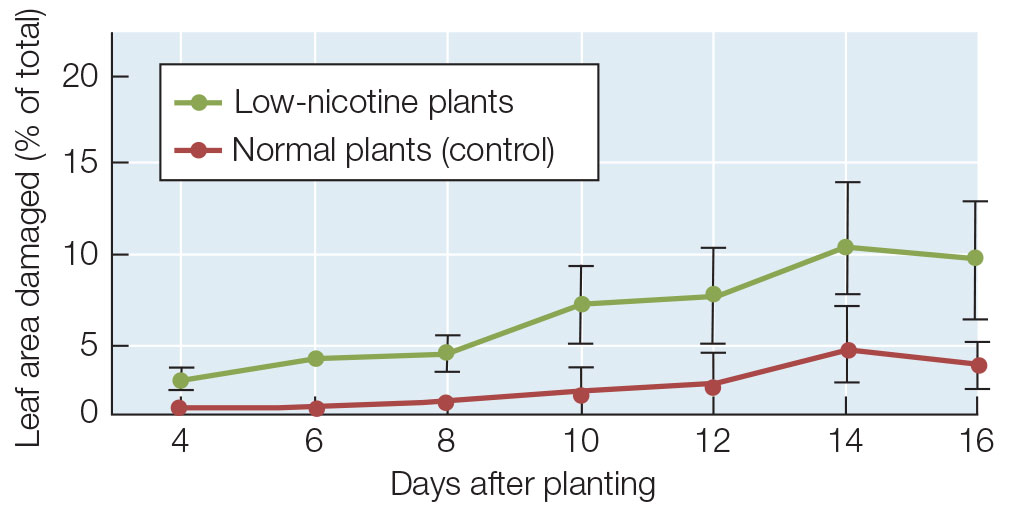
While the mechanisms of action of secondary metabolites and their presence in plant tissues are circumstantial evidence of their role in defense, experiments can be done to provide cause-and-effect evidence. Nicotine, for example, kills insects by acting as an inhibitor of nervous system function, and its function in tobacco is presumed to be insecticidal. Yet commercial varieties of tobacco and related plants that produce nicotine are still attacked, with moderate damage, by pests such as the tobacco hornworm (see Figure 28.3B). Given that observation, does nicotine really deter herbivores? Biologists answered this question conclusively with a study that used tobacco plants in which an enzyme involved in nicotine biosynthesis had been inhibited, lowering the nicotine concentration in the plants by more than 95 percent. These low-nicotine plants suffered much more damage from insect herbivory than normal plants did (FIGURE 28.4).
Investigation
HYPOTHESIS
Nicotine helps protect tobacco plants against insects.
METHOD
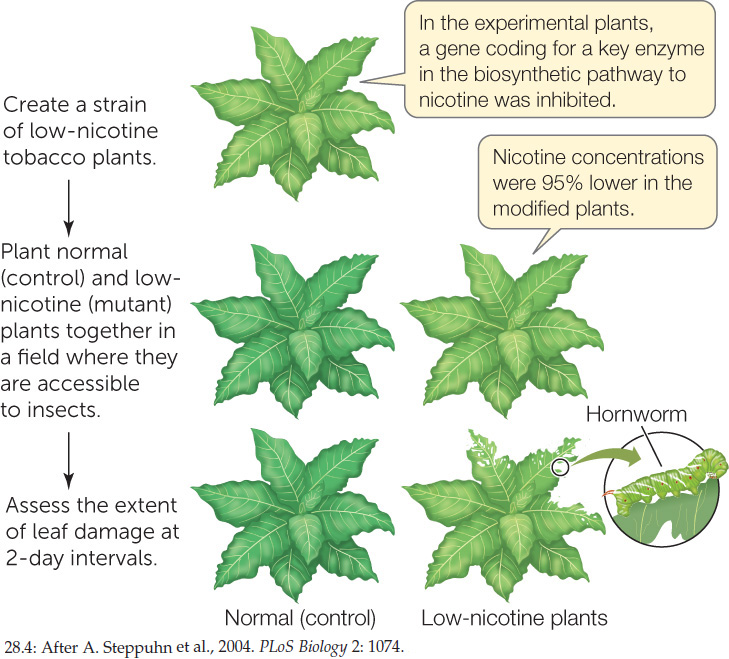
RESULTS
The low-nicotine plants suffered more than twice as much leaf damage as did the normal controls.
CONCLUSION
Nicotine provides tobacco plants with at least some protection against insects.
ANALYZE THE DATA
In a separate experiment, Baldwin and his colleagues showed that treatment with jasmonic acid (jasmonate) increased the concentration of nicotine in normal tobacco plants but not in the low-nicotine plants. The researchers planted a group of normal and low-nicotine plants and treated them with jasmonate 7 days after planting. The plants were assessed for herbivore damage every 2 days after being planted. The results are shown in the graph.
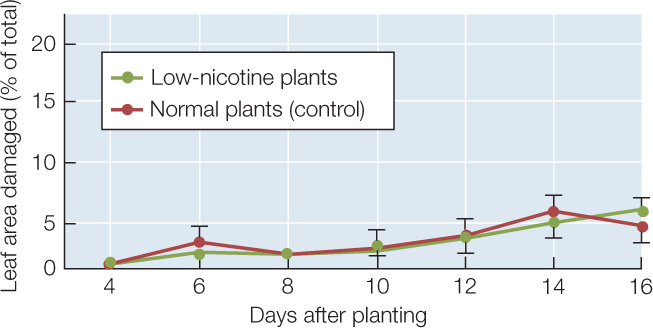
- Compare these data to those above for the untreated plants. What was the effect of jasmonate treatment on the resistance of normal plants to herbivore damage? What was the effect on low-nicotine plants?
- What do these data reveal about the role of nicotine in preventing herbivore damage?
- Explain how jasmonate could have had the effect it did on the low-nicotine plants, even though their nicotine levels were still low after jasmonate treatment.
- The error bars at each data point are the standard error of the mean (SEM). What statistical test (see Appendix B) would you use to determine the possible significance of differences between the jasmonate-treated and untreated plants? At 10 days, the mean damage ± SEM for untreated low-nicotine plants was 6.0 ± 1.5 % (n = 36), and for treated low-nicotine plants it was 2.2 ± 0.6 % (n = 28). Run a statistical test comparing the two results, calculate the P-value, and comment on significance.
Go to LaunchPad for discussion and relevant links for all INVESTIGATION figures.
aP. Steppuhn et al. 2004. PLOS Biology 2: e217.
Plants respond to herbivory with induced defenses
The first step in a plant’s response to an attack by an herbivore is to sense the attack, either by means of changes in membrane potential or by means of chemical signals. The detection of an herbivore attack then triggers signal transduction pathways that induce plant defenses.
Membrane Signaling
The cell membrane is the part of the plant cell that is in contact with the environment. Within the first minute after an herbivore strikes, changes in the electric potential of the cell membrane occur in the damaged area. The continuity of the symplast (see Concept 25.3) ensures that the signal travels over much of the plant within 10 minutes.
LINK
In animals, direct electrical coupling between cells occurs only in specific tissues, such as cardiac muscle; see Concept 33.1
Chemical Signaling
When some insects (such as caterpillars) chew on a plant, substances in the insect’s saliva combine with fatty acids derived from the consumed plant tissue. The resulting compounds act as elicitors to trigger both local and systemic responses to the herbivore. In corn, the elicitor produced by one particular moth larva has been named volicitin for its ability to induce production of volatile signals that can travel to other plant parts—and to neighboring corn plants—and stimulate their defensive responses.
595
Signal Transduction Pathway
The perception of damage from herbivory can initiate a signal transduction pathway in the plant that involves the plant hormone jasmonic acid (jasmonate) (FIGURE 28.5; also see Figure 28.4, Analyze the Data):
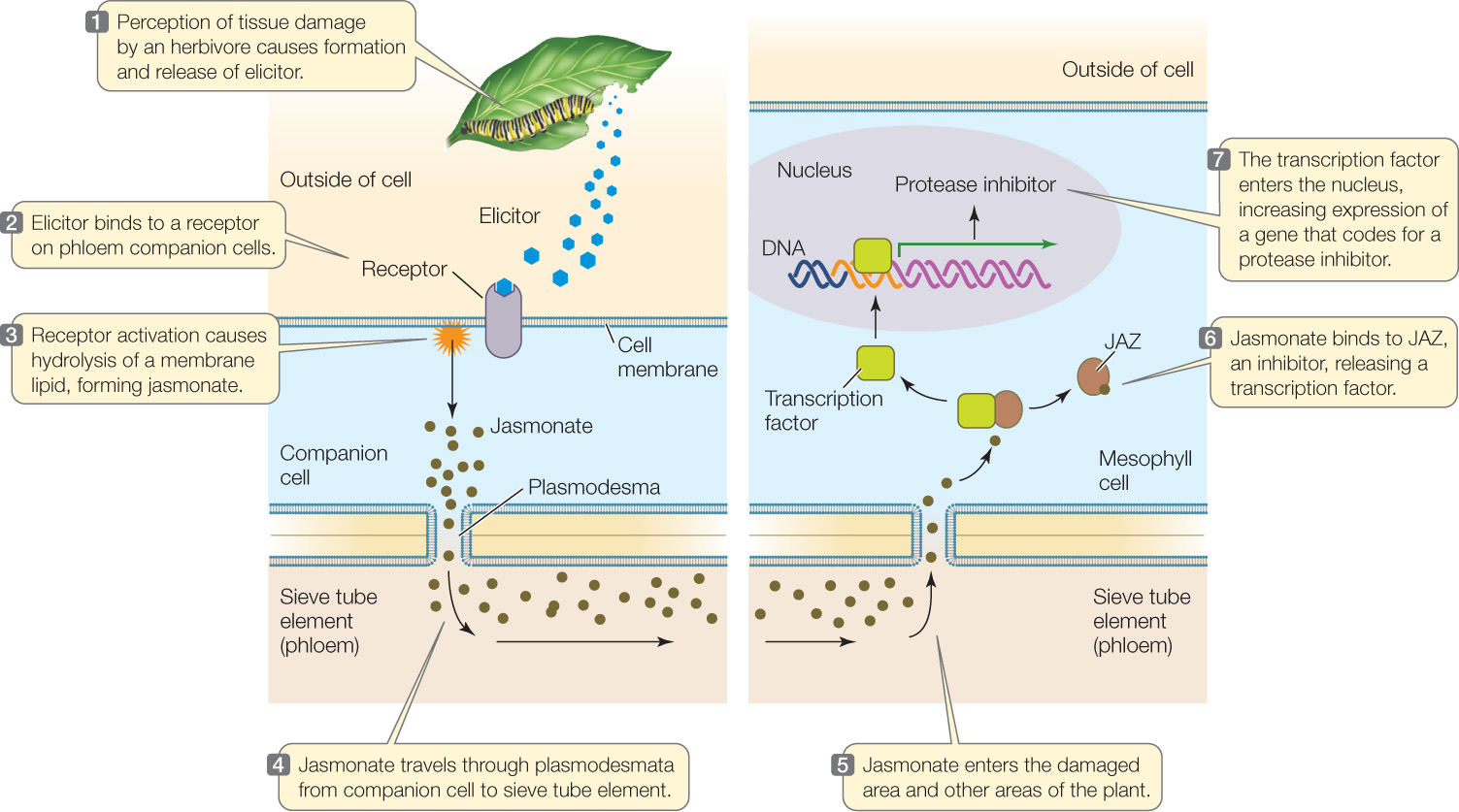
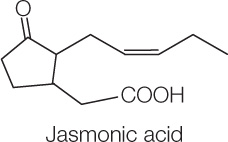
When the plant senses an herbivore-produced elicitor, it makes jasmonate, which triggers many plant defenses, including the synthesis of protease inhibitors. The inhibitors, once in an insect’s gut, interfere with the protease-catalyzed digestion of proteins and thus stunt the insect’s growth. Jasmonate also “calls for help” by triggering the formation of volatile compounds that attract insects that prey on the herbivores attacking the plant. Similarly, the volatile compounds induced by volicitin in corn attract wasps that parasitize herbivorous caterpillars, so the “call for help” is a recurrent theme in plant–insect interactions.
596
Why don’t plants poison themselves?
Why don’t the constitutive defensive chemicals that are so toxic to herbivores and pathogens kill the plants that produce them? Plants that produce toxic defensive chemicals generally use one of several measures to protect themselves.
Compartmentalization
Isolation of the toxic substance is the most common means of avoiding exposure. Plants store their toxins in vacuoles if the toxins are water-soluble. If they are not water-soluble, the toxins may be dissolved in latex (a complex mixture of dissolved molecules and suspended particles) and stored in a specialized compartment, or they may be dissolved in waxes on the epidermal surface. Such compartmentalized storage keeps the toxins away from mitochondria, chloroplasts, and other parts of the plant’s metabolic machinery.
LINK
Both plants and animals use compartmentalization to isolate toxins and waste products that occur as by-products of some biochemical reactions; see Concept 4.3
Storage of Precursors
Some plants store the precursors of toxic substances in one type of tissue, such as the epidermis, and store the enzymes that convert those precursors into the active toxin in another tissue, such as the mesophyll. When an herbivore chews part of the plant, cells are ruptured, the enzymes come into contact with the precursors, and the toxin is produced. The only part of the plant that is damaged by the toxin is that which was already damaged by the herbivore. Plants such as sorghum and some legumes, which respond to herbivory by having molecules that produce cyanide when ingested by animals, are among those that use this type of protective measure. Cyanide is a potent inhibitor of cellular respiration, and when people eat such plants, they first cook them or otherwise prepare them (e.g., soaking, fermenting, drying) to inactivate the cyanide-producing molecules.
Modified Proteins
Some plants have modified proteins that do not react with the toxin. As noted above, canavanine resembles arginine and therefore plays havoc with protein synthesis in insect larvae. In plants that make canavanine, the enzyme that catalyzes bond formation between arginine and its counterpart tRNA molecule discriminates correctly between arginine and canavanine, so canavanine is not incorporated into the plant’s proteins.
Plants don’t always mount a successful defense
Milkweeds such as Asclepias syriaca store their defensive chemicals in latex in specialized tubes called laticifers, which run alongside the veins in the leaves. When damaged, a milkweed releases copious amounts of toxic latex from its laticifers. Field studies have shown that most insects that feed on neighboring plants of other species do not attack laticiferous plants, but there are exceptions. One population of beetles that feeds on A. syriaca exhibits a remarkable prefeeding behavior: these beetles cut a few veins in the leaves before settling down to dine. Cutting the veins causes massive latex leakage from the adjacent laticifers and interrupts the latex supply to a downstream portion of the leaf. The beetles then move to the relatively latex-free portion and eat their fill.
597

This is just one of many ways that herbivores circumvent plant defenses. A successful plant defense exerts strong selection pressure on herbivores to get around it somehow; a successful herbivore, in turn, exerts strong selection pressure on plants to develop new defensive strategies. One can imagine, for example, that over time A. syriaca might evolve to have thicker walls around the base of its laticifers, such that the beetles can no longer cut them, or to produce a different toxin that does not depend on laticifers.
LINK
You can find more examples of the “evolutionary arms race” between plants and herbivores in Concept 43.4; see especially Figures 43.10 and 43.11
CHECKpoint CONCEPT 28.2
- Using a plant growing near you as an example, describe anatomical features that help plants avoid herbivores.
- A mutant strain of a plant makes jasmonate constitutively. What would be the herbivory defense phenotype of this plant?
- You have isolated a secondary metabolite from apple tree leaves. What circumstantial and experimental evidence would you use to show that the metabolite prevents the herbivorous larva of the moth Spilonota ocellana from damaging apple orchards?
In addition to coping with their biological enemies, plants must survive in their physical environment. Plants have a number of responses that allow them to cope with stressful environmental conditions.