Concept 35.2: Hormones Are Chemical Messengers Distributed by the Blood
In Chapter 34 we discussed the nervous system in detail. Now we will turn to a detailed discussion of the endocrine system, starting by defining its fundamental properties.
Animals have two types of glands, which are termed exocrine and endocrine. All glands produce and secrete materials. The exocrine glands typically have outflow tubes, called ducts, that carry their secretions away. Examples include the mammary, salivary, and tear glands. In each case, the gland secretes a material (milk, saliva, or tears) that flows out by way of tubular ducts. The prostate gland, which contributes fluid to the semen, is another exocrine gland.
The endocrine glands do not have ducts (and therefore are sometimes called ductless glands). Endocrine cells, by definition, secrete their products into the blood flowing through nearby blood capillaries or other blood passages (keep in mind that in this book we use the term blood to refer to the fluid circulating in the circulatory system in all animals; see Concept 32.1). Because endocrine cells secrete into the blood, endocrine glands require only a blood supply, not ducts, for their secretions to be carried away.
In some cases, endocrine cells exist as single cells scattered within a tissue composed mostly of other cells, as we have observed in the gut epithelium (see Concept 30.5). In other cases, endocrine cells are aggregated (grouped) together to form discrete tissues or organs. These are the endocrine glands. Examples include the thyroid gland and adrenal glands.
The testes, ovaries, and pancreas illustrate a complexity that sometimes arises: these organs are composed of a mix of endocrine and non-endocrine tissues. A vertebrate testis, for example, consists mostly of tiny, non-endocrine tubules in which sperm are produced. In between these tubules are small aggregations of endocrine cells called the Leydig cells or interstitial cells. The testes are sometimes called endocrine glands. Technically, however, they are only partially endocrine glands.
A hormone is defined to be a chemical substance that is secreted into the blood by endocrine cells and that regulates the function of other cells that it reaches by blood circulation. An additional defining feature of hormones is that they act at very low blood concentrations: sometimes as low as 10−12 molar (moles per liter). Still another defining feature is that the effects of hormones on target cells are initiated by noncovalent binding of the hormone molecules to receptor protein molecules synthesized by the target cells. This feature gives hormones their specificity of action, as we stressed before. The specific action of a hormone on a target cell depends on the receptor proteins that the cell synthesizes and on the biochemical pathways the receptor proteins activate.
Growth, development, reproductive cycles, water balance, and long-term stress responses are some of the processes that typically are under primarily hormonal control in animals. This list reflects the fact that, as we discussed in Concept 35.1, hormones are well suited to control processes that involve many tissues and that occur on time scales of hours, days, months, or years.
Endocrine cells are neurosecretory or nonneural
Two broad classes of endocrine cells exist (FIGURE 35.3). Some, called neurosecretory cells or neuroendocrine cells, resemble neurons in that they are excitable cells that propagate action potentials. Others, called nonneural endocrine cells or epithelial endocrine cells, are not excitable.
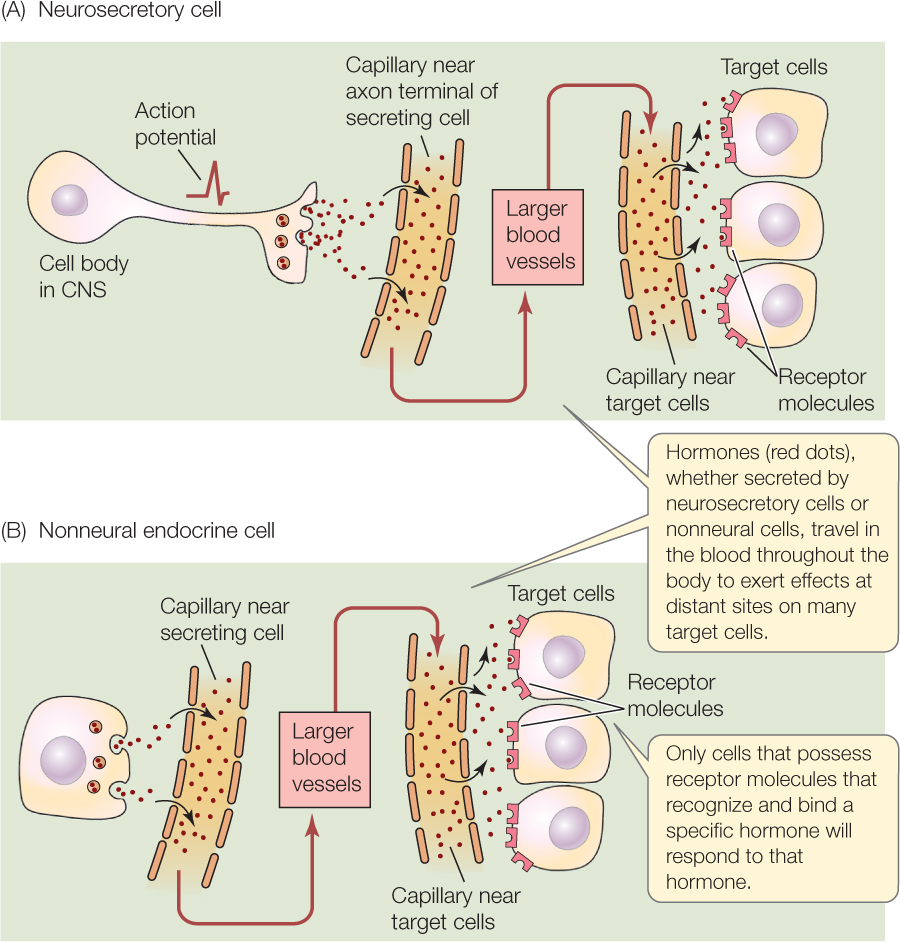
The cell bodies of neurosecretory cells (see Figure 35.3A) are located in the central nervous system (CNS). These cells have axons, which typically extend outside the CNS. Neurosecretory cells release hormones into the blood at their axon terminals, instead of releasing neurotransmitters at synapses as neurons do. Often, groups of neurosecretory cells have their axon terminals positioned within a neurohemal organ (neuro, “related to neurons”; hemal, “related to blood”), a specialized part of the circulatory system in which the terminals are closely juxtaposed with the blood.
Neurosecretory cells provide a direct interface between the nervous and endocrine systems. Ordinary neurons make synaptic contact with the dendrites and cell body of a neurosecretory cell in the CNS. The neurosecretory cell responds to neural activity in the CNS by secreting a hormone into the blood. The typical order of events in the function of a neurosecretory cell is that neurons in the CNS activate the cell by exciting it at synapses. The neurosecretory cell, in response, initiates and propagates action potentials. These action potentials activate hormone release into the blood at the cell’s axon terminals. An example is provided by the cells that produce the hormone oxytocin in mammals. Oxytocin plays critical roles during the birth process and during nursing. It is secreted by neurosecretory cells in the hypothalamus of the brain.
737
The secretions of neurosecretory cells can quite properly be called hormones. Sometimes, however, scientists distinguish these secretions by calling them neurohormones or neurosecretions.
Nonneural endocrine cells (see Figure 35.3B) do not employ action potentials. They are typically stimulated to secrete their hormones by other hormones, for which they express receptor proteins. The typical order of events in the function of a nonneural endocrine cell is that the cell receives a hormonal signal from another gland. This hormone binds to receptor proteins, which initiate secretion by the nonneural endocrine cell. The cells of the anterior pituitary gland and thyroid gland provide examples.
Most hormones belong to one of three chemical groups
There is enormous diversity in the chemical structure of hormones, but we can classify most hormones into one of three groups:
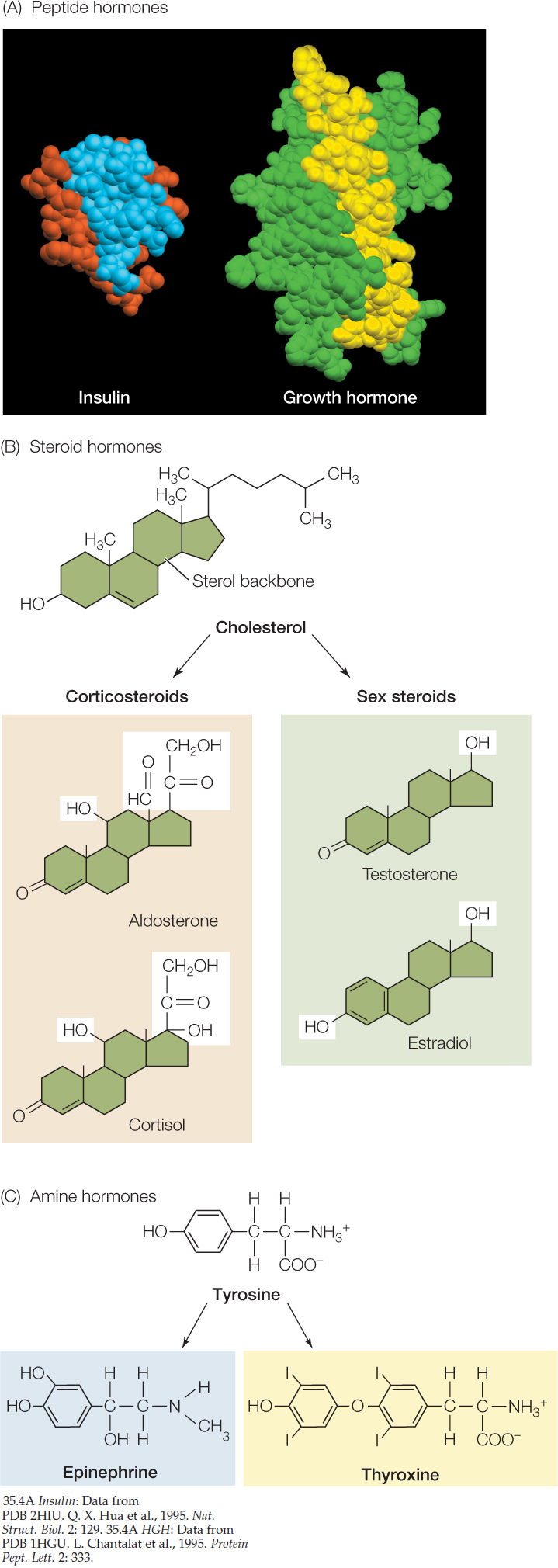
- Peptide and protein hormones are molecules composed of chains of amino acids. They are water-soluble and therefore easily transported in the blood. Most hormones are in this group. Some are large molecules that may contain nearly 200 amino acid units (FIGURE 35.4A). Others are small molecules containing as few as three amino acid units. For simplicity, these hormones are often all referred to as peptide hormones (disregarding the fact that the larger ones are technically proteins), and we follow that practice here. During their synthesis, the peptide hormones are packaged in vesicles within the cells that make them. They are released by exocytosis (see Concept 5.4). Because water-soluble molecules cannot easily cross cell membranes, the receptors for peptide hormones are on the exterior of a target cell. There are dozens, perhaps hundreds, of peptide hormones, with diverse structures and effects.
- Steroid hormones are synthesized from cholesterol and share a similar structure of four interlinked rings (FIGURE 35.4B). Steroid hormones are lipid-soluble and readily pass through the membranes of the cells that synthesize them, but they do not dissolve well in blood plasma. Thus they are usually bound to carrier proteins for transport in the blood. Once they reach a target cell, they typically diffuse directly through its cell membrane. Most steroid receptors are found inside target cells, in the cytoplasm or nucleus.
- Amine hormones are small molecules that are modified amino acids. For example, both the thyroid hormone thyroxine and the fight-or-flight hormone epinephrine are made from the amino acid tyrosine (FIGURE 35.4C), and the hormone melatonin (which affects an animal’s daily rhythms) is made from tryptophan. Depending on whether the modified amino acid is polar or nonpolar, an amine hormone may be water-soluble (can’t readily cross membranes) or lipid-soluble (can readily cross membranes).
Receptor proteins can be on the cell surface or inside a cell
Some receptor proteins are positioned in the cell membrane of a target cell, as in the case of the receptors for peptide hormones and some amine hormones. The hormone binds to a part of the receptor protein that projects outside the cell membrane, and then the receptor molecule initiates processes that alter cell function. Many receptors of this type are G protein–linked receptors (see Concept 5.5) that, after binding to a hormone, initiate second messenger cascades inside the target cell. These cascades activate or inactivate enzymes in the cell cytoplasm, leading to the cell’s response (see Figure 5.14).
738
Other receptor proteins are located inside the target cell, in the cytoplasm or nucleus. This is the case for the receptors for steroid hormones and some amine hormones. These hormones can diffuse through the cell membrane because they are lipid-soluble. When a steroid hormone binds to its receptor in the cytoplasm, the hormone–receptor complex usually moves to the cell nucleus. There it alters gene expression (transcription and translation), resulting in synthesis of new proteins. For example, when testosterone binds to receptors in the cytoplasm of skeletal muscle cells, testosterone–receptor complexes move into the nuclei of the cells and activate transcription of several genes. These genes include those that code for enhanced synthesis of the contractile proteins actin and myosin (see Concept 33.1). In this way, testosterone tends to bring about an increase in muscle mass.
LINK
You can learn more about the various types of receptor proteins in Concept 5.5
A target cell is not limited to expressing only a single kind of hormone receptor protein. Cells often express two or more receptor proteins, which make them target cells for two or more hormones.
The number of receptor molecules that a target cell has for a particular hormone can vary. In some cases, negative or positive feedback affects the number of receptor molecules that a target cell expresses. For example, continuous high concentrations of a hormone sometimes cause a target cell to decrease its number of receptors for the hormone. This decrease makes the cell less sensitive to the hormone, tending to offset the effect of the continuous high concentration. For example, in type II diabetes mellitus, chronically high levels of the hormone insulin (usually caused by excessive carbohydrate intake) lead to decreased production of insulin receptors throughout the body. As a result, cells become less sensitive to insulin.
LINK
The crucial role of insulin in regulating glucose metabolism is detailed in Concept 30.5
739
Hormone action depends on the nature of the target cells and their receptors
The action of a hormone on a target cell depends on characteristics of the target cell—in particular, the receptor protein the cell expresses and the cellular processes activated by the receptor protein. For this reason, one hormone can have dramatically different effects on different cells.
In the course of evolution, many hormones have been conserved for long periods of evolutionary time, but their functions have changed as various types of animals have evolved. This pattern—in which a single hormone molecule evolves changing functions—arises because the actions of a hormone depend on the receptor systems, which can change. The peptide hormone prolactin provides a dramatic example.
Prolactin was first discovered in mammals, where it stimulates milk synthesis in the mammary glands (breasts). Prolactin has since been found in all other vertebrate groups—from fish to birds—even though these other phyla have neither mammary glands nor milk. In salmon, prolactin is involved in stabilizing blood ion composition as the fish migrate between salt and fresh water. In birds, prolactin can stimulate nest building and parental care. The hormone—in one molecular form or another—has existed in vertebrates throughout their evolutionary history, but prolactin has taken on radically different functions in various vertebrate groups by evolution of the target cells it affects and their receptor systems.
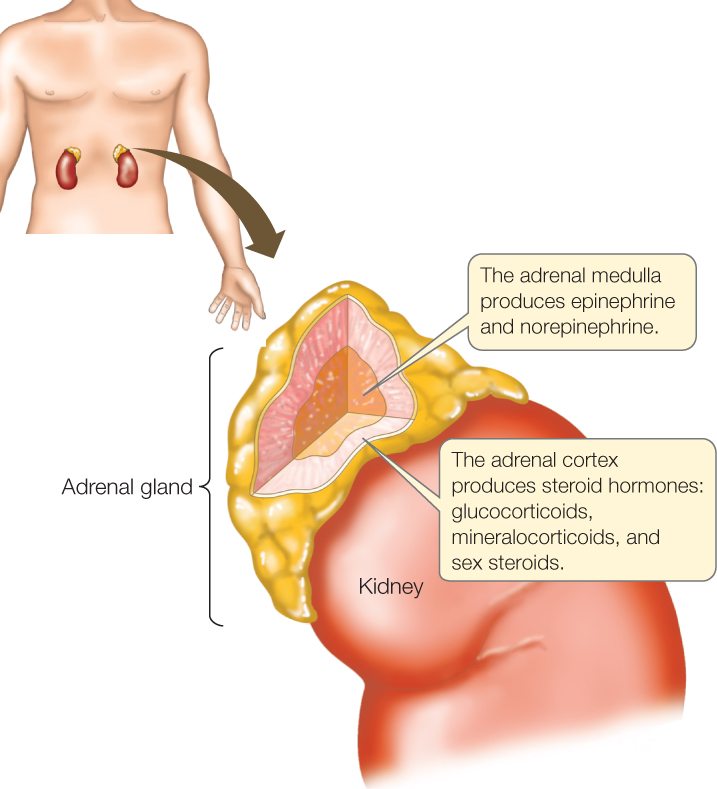
Within one individual animal, some target cells for a hormone can have different receptor proteins and receptor systems than other target cells do. The hormone then elicits different responses from the different types of target cells. For example, in a sudden emergency, our fight-or-flight response is activated to help us confront the emergency. As part of this response, our adrenal medullary glands (FIGURE 35.5) secrete epinephrine and norepinephrine (sometimes known as adrenaline and noradrenaline), while at the same time our sympathetic nervous system (see Concept 34.5) exerts its effects. Within seconds, these hormones are circulating in our blood. We have at least five types of receptors for the hormones, all of them G protein–linked receptors. These receptors fall into two major categories—called α-adrenergic and β-adrenergic receptors—that bind epinephrine and norepinephrine with different degrees of ease. Cells of different tissues have different receptors. Moreover, even when different types of cells have the same receptor, they may respond very differently because the receptor activates different processes in different cell types. For these reasons, the hormones bring about a large range of responses in various tissues: faster and stronger beating of the heart, constriction of blood vessels in the skin, dilation of blood vessels in skeletal muscles, breakdown of glycogen to release glucose in the liver (see Figure 5.17), and a decrease in both blood flow and secretion of digestive enzymes in the gut.
LINK
Signal transduction pathways—the cellular processes activated by receptors—are discussed in Concept 5.6
A hormonal signal is initiated, has its effect, and is terminated
When endocrine cells are activated to secrete a hormone, they vary in how quickly they start hormone release. Peptide and amine hormones are usually synthesized prior to use. They can be secreted very rapidly because they are present in stored form in endocrine cells. In contrast, steroid hormones are typically synthesized on demand. Initiation of secretion of steroid hormones is relatively slow because of the need to synthesize the hormones before they can be released.
Just as there are processes for secreting a hormone into the blood, there are ways to remove it. Removal takes place in several ways. Target cells sometimes enzymatically degrade hormones. Certain organs, such as the liver and kidneys in vertebrates, also enzymatically degrade hormones. Hormones may also be excreted.
The removal processes ultimately terminate a hormone signal. The half-life of a hormone in the blood is the time required for half of a group of simultaneously secreted hormone molecules to be removed from the blood. Some hormones have half-lives measured in minutes, and in these cases an individual hormonal signal does not last long. Epinephrine, for example, has a half-life of 1–2 minutes, explaining why the jolt of a fight-or-flight response disappears quickly if we decide no emergency exists and we are safe. Other hormones have far longer half-lives. In the human bloodstream, for example, the hormones antidiuretic hormone (involved in water conservation), cortisol (involved in long-term responses to stress), and thyroxine (affects cellular metabolism) display average half-lives of about 15 minutes, 1 hour, and nearly 1 week, respectively. They may therefore have prolonged effects on target tissues.
When a hormone is secreted steadily over a period of time, the blood hormone concentration is determined by an interaction between the rate of secretion (adding hormone to the blood) and the rate of removal. Removal of any particular hormone tends to take place at a fairly steady rate. Thus changes in the blood concentration of a hormone depend chiefly on changes in the rate at which the hormone is secreted.
740
APPLY THE CONCEPT: Hormones are chemical messengers distributed by the blood
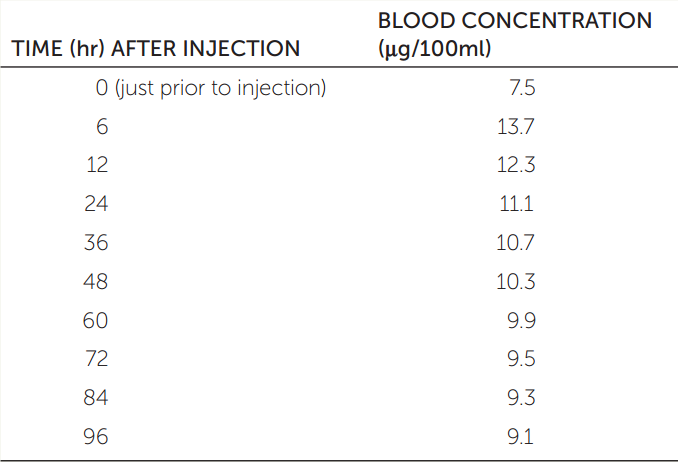
One way to characterize the time course of a hormone is to measure its half-life in the blood. A hormone’s half-life can be measured in the laboratory by injecting some of the hormone and then determining the length of time required for the blood level of the hormone to fall from its maximum level halfway back to its baseline value. The table gives blood concentrations of the thyroid hormone thyroxine (T4) following a 600-μg injection in a particular experiment. Plot these data.
- Use the data in the table to estimate the half-life of 600 μg of T4 in the bloodstream in this experiment. (The concentration at time 0 is the baseline.)
- If you were trying to correct a hormone deficiency by administering hormone therapy and needed to keep the hormone level in the blood from falling too low, how would your dosing frequency differ for hormones with different half-lives?
The blood concentration is stabilized by negative feedback in many cases, making negative feedback a major theme in the study of endocrinology. During negative feedback, if the blood concentration rises above a set-point level, secretion of the hormone is inhibited.
LINK
Many physiological processes in animals have set points and are regulated by negative feedback; Concept 29.6 describes the components of negative-feedback systems
When they are in the blood, some hormones are bound to carrier proteins. For example, steroid hormones must be bound with water-soluble proteins to be in solution in the blood. Carrier proteins often extend the half-lives of hormones.
Some hormones are converted to more active forms after they are secreted, a process called peripheral activation. A classic example is provided by the hormones of the thyroid gland. The thyroid secretes a molecule that contains four iodine atoms. This molecule is called thyroxine, T4, or tetraiodothyronine (see Figure 35.4C). The hormone becomes more active when peripheral tissues enzymatically remove one of the iodine atoms, forming T3, triiodothyronine.
CHECKpoint CONCEPT 35.2
- Growth hormone is a large, water-soluble peptide. Given these characteristics, how would you expect this hormone to interact with its target cells?
- Are sweat glands correctly described as endocrine glands?
- Name one major similarity and one major difference between an ordinary neuron and a neurosecretory cell.
For understanding the vertebrate endocrine system, one of the most important themes is that the brain often controls the secretion of hormones by the endocrine system. We will start with that theme as we now turn our attention to the vertebrate endocrine system.