Concept 45.3: Certain Biogeochemical Cycles Are Especially Critical for Ecosystems
All biogeochemical cycles can be described in terms of compartments containing the material involved, amounts (pools) of the material contained in each compartment, and rates (fluxes) at which the material enters and leaves those compartments (see Figure 45.5). At the same time, the cycles of different materials vary in their spatial scales and in the processes that drive them. We will illustrate this variation by tracing the cycles for three essential materials: water, nitrogen, and carbon. We will point out how each cycle affects the global ecosystem, particularly the atmosphere, and is in turn affected by human activities.
Water transports materials among compartments
Water is a most remarkable molecule. It makes up more than 70 percent of living biomass. It is the medium for metabolism and the solvent in which biologically accessible forms of many nutrients are dissolved. Precipitation of water as rain or snow transports materials from the atmosphere to Earth’s surface. Water weathers rock into soil. And as the primary agent of erosion and transport of dissolved ions and sediment, water is responsible for much of the physical movement of materials around the planet. By virtue of its high heat capacity and its ability to change between solid, liquid, and gaseous states at normal Earth temperatures, water redistributes heat around the planet as it circulates through the oceans and atmosphere. And, as we will soon see, water in the atmosphere is an important player in the global radiation balance.
LINK
The molecular nature of water and its crucial role in the evolution of life as we know it are covered in Chapter 2
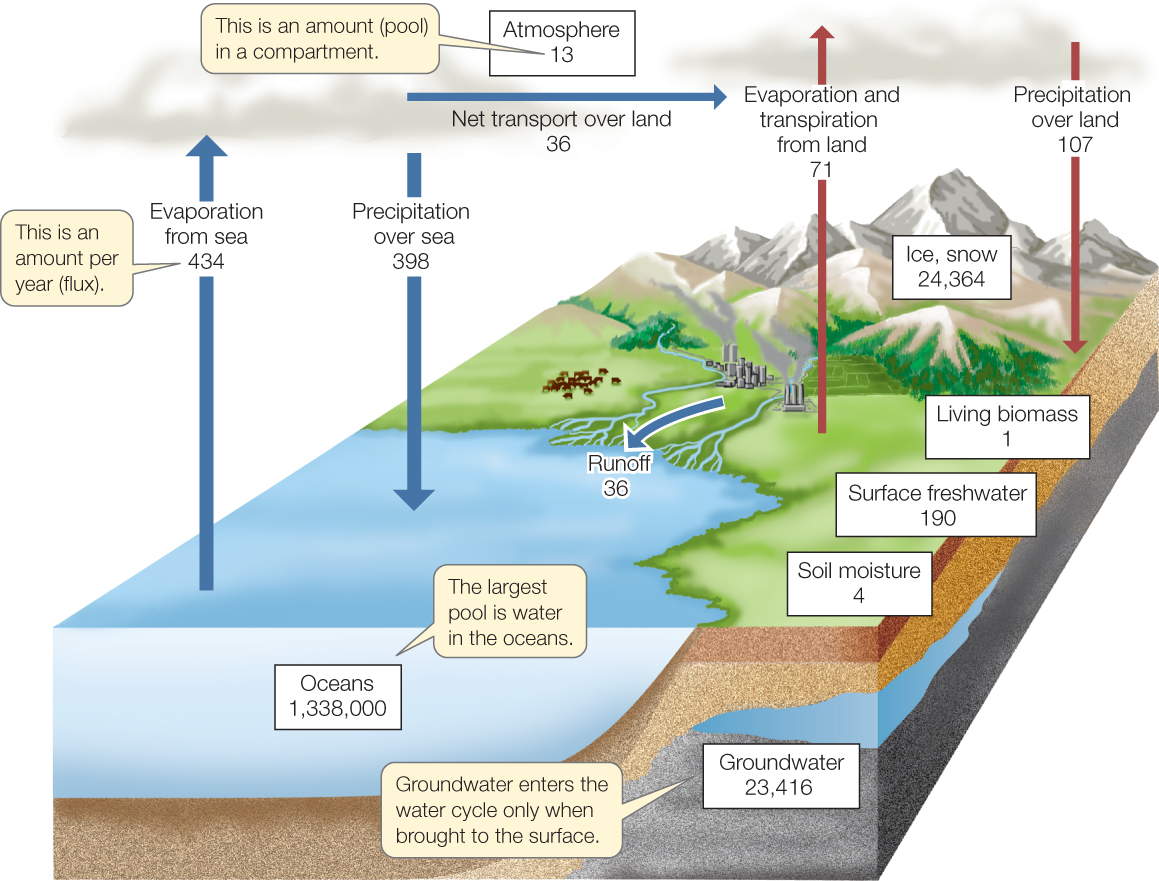
The global ecosystem contains almost 1.4 billion cubic kilometers of water. About 96.5 percent of this water is in the oceans, which cover 71 percent of the planet’s surface—making it obvious why Earth is sometimes called the “blue planet.” Other compartments of water include ice and snow (1.76%), groundwater (1.70%), the atmosphere (0.001%), and fresh water in lakes and rivers (surface fresh water; 0.013%). Even though biomass is mostly water, only about 0.0001 percent of Earth’s water is found in living organisms.
Some fluxes among these compartments (FIGURE 45.6) involve gravity-driven flows of water from the atmosphere to Earth’s surface (precipitation) and from land to the oceans and other bodies of water (runoff). Other fluxes, however, are associated with changes in the physical state of water—from liquid to gas (evaporation), from gas to liquid (condensation), between liquid and solid (freezing and melting), and between solid and gas (sublimation and deposition).

Go to ANIMATED TUTORIAL 45.1 The Global Water Cycle
PoL2e.com/at45.1
The driving force of the water cycle is solar-powered evaporation, which transforms water from liquid to gas and, in the process, moves it into the atmosphere from oceans and other bodies of water, from the soil, and from plants (via transpiration; see Concept 25.3) and other organisms. It takes considerable energy—2.24 kJ (about the energy stored in one AA battery)—to evaporate 1 gram of water. On a global scale, the rate of evaporation is enormous, taking up about one-third of the total solar energy that reaches Earth’s surface. This energy is released again as heat when the water vapor condenses to form precipitation. Fluxes of water from Earth’s surface to the atmosphere and from the atmosphere to the surface are approximately equal on a global basis, although precipitation exceeds evaporation over land, and evaporation exceeds precipitation over the oceans.
Humans affect the global water cycle when they change the way land is used. Removing vegetation reduces the amount of precipitation that is retained by soil or groundwater and recycled locally, thereby increasing the amount that leaves the local ecosystem as surface runoff. As a result, deforestation, grazing, and crop cultivation all tend to dry out local ecosystems. Pumping depletes groundwater, moving it to the surface, where it either evaporates or runs off into streams and, eventually, into oceans.
922
The water cycle also affects and is affected by the global climate. Warming of the climate is melting polar ice caps and glaciers, increasing the total amount of water in the oceans and causing sea level to rise. With more liquid water comes more evaporation, and thus more water entering the atmosphere and more precipitation globally. Water vapor absorbs infrared radiation—and thus contributes to the greenhouse effect—but it also forms clouds, which reflect incoming sunlight back into space. Scientists are working to predict the net effects on climate of these counterbalancing changes in the water cycle.
Within-ecosystem recycling dominates the global nitrogen cycle
Nitrogen is abundant on Earth, yet it is often in short supply in biological communities. Unlike water—whose availability to organisms depends only on where it occurs and its physical state—nitrogen’s accessibility to life depends not only on where it occurs, but also on what other elements it is attached to. As a result, the nitrogen cycle, unlike the water cycle, involves chemical transformations (FIGURE 45.7).


Go to ANIMATED TUTORIAL 45.2 The Global Nitrogen Cycle
PoL2e.com/at45.2
The gas N2 constitutes about 78 percent of the molecules in Earth’s atmosphere, but most organisms cannot use nitrogen in this form because they cannot break the strong triple bond between the two nitrogen atoms. The exceptions are certain microbes that break the triple bond and attach hydrogen to make ammonium (NH4+)—which is usable by other organisms—through a metabolic process called nitrogen fixation. In terrestrial ecosystems, nitrogen fixation is carried out mostly by free-living bacteria in the soil and by symbiotic bacteria associated with plant roots, but it also occurs in other places, such as the fungal gardens of leaf-cutter ants described in the opening story of Chapter 43 (FIGURE 45.8). Analogous biochemical processes in aquatic ecosystems are carried out by free-living microorganisms and by microbial symbionts of phytoplankton and other aquatic organisms. A small amount of nitrogen is fixed in the atmosphere by lightning. Diverse other microbial species in terrestrial and aquatic ecosystems convert ammonium into other accessible forms of nitrogen such as nitrate (NO3−). Nitrogen fixation is reversed by another group of microbes in a process called denitrification, which ultimately completes the cycle by returning N2 gas to the atmosphere.
Investigation
HYPOTHESIS
Nitrogen-fixing organisms in leaf-cutter ant nests supply nitrogen to the ants.
METHOD
- Bring ant colonies into the laboratory and allow them to function in an environment with no other insects and no soil (that is, where the only non-atmospheric source of N is fresh leaves supplied by the researchers).
- Measure the nitrogen content of leaves, fungus garden, ants, and leaf refuse.
RESULTS

Error bars indicate 1 standard error from the mean; bars with different letters are statistically different from one another. (See Appendix B for discussion of statistical concepts.)
CONCLUSION
Nitrogen is being fixed within the ant nest.
ANALYZE THE DATA

The researchers measured activity of nitrogenase, an enzyme in the bacterial metabolic pathway that fixes atmospheric nitrogen. The results are shown in the figure to the left. Error bars indicate 1 standard error from the mean; different letters indicate significant differences in nitrogenase activity.
- Where in the nest is nitrogen fixation occurring?
- How can the ant workers have a higher nitrogen content than the fungus garden (see figure above)?
- Can the researchers now conclude that the extra nitrogen comes from nitrogen-fixing bacteria living in the fungus gardens?
Go to LaunchPad for discussion and relevant links for all INVESTIGATION figures.
aA. A. Pinto-Tomás et al. 2009. Science 326: 1120–1123.
LINK
The metabolic processes by which microbes transform nitrogen are described in Concepts 19.3 and 25.2
Ammonium and nitrate are accessible to autotrophs because they dissolve in water and thus are easily taken up through cell membranes. Heterotrophs obtain nitrogen by breaking proteins down into amino acids, which can also be transported through cell membranes. Much of the nitrogen in terrestrial ecosystems is recycled locally as decomposition of dead organic matter releases accessible nitrogen into the soil. Some nitrogen, however, is lost to the atmosphere when biomass burns, or is leached from soils and carried away in streams, ultimately into lakes and oceans. In aquatic systems, primary production occurs in surface waters, and some nitrogen is recycled there. However, much of it is recycled at greater depths in the water column as organisms in deep waters intercept and consume sinking organic matter. The nitrogen in this organic matter eventually accumulates in sediments. Some of it is returned to the surface by upwelling or, eventually, by tectonic processes followed by weathering of exposed rock.
923
Human activities are affecting nitrogen fluxes and pools. Cultivation of wetland crops (such as rice), livestock raising, and burning of plant material, coal, and petroleum release oxides of nitrogen and ammonia into the atmosphere. These molecules contribute to atmospheric smog and acid rain, and one of them, nitrous oxide (N2O), absorbs infrared radiation. When they are eventually deposited on Earth’s surface, sometimes far from their source, they can add as much nitrogen “fertilizer” as farmers place on their crops. Humans also alter the atmospheric pool of N2 through the industrial process that fixes nitrogen to manufacture fertilizer and explosives. The rate of industrial fixation (see Concept 25.1) plus the rate of fixation by legume crops such as soybeans now adds up to more than the rate of natural terrestrial nitrogen fixation (see Figure 45.7). Transport of topsoil and dissolved nitrogen from fertilized croplands and deforested areas by wind or water runoff is increasingly exporting nitrogen from terrestrial to aquatic ecosystems.
Increased nitrogen inputs can increase primary production, but too much of a good thing can be a bad thing. In aquatic systems, nutrient-stimulated increases in primary productivity—a phenomenon called eutrophication—can result in rapid growth of phytoplankton (including cyanobacteria; see Figure 19.9C) and algae. Respiration by these primary producers and by the decomposers that process their dead bodies can reduce oxygen levels below the tolerances of many aquatic organisms, including fish and crustaceans. Eutrophication can lead to “dead zones” devoid of oxygenrequiring aquatic life, like that found where the Mississippi River discharges nutrient-rich agricultural runoff into the Gulf of Mexico (FIGURE 45.9). In nutrient-poor terrestrial ecosystems, excess nitrogen can change the species composition of plant communities. Species that are adapted to low nutrient levels grow slowly, even when fertilized, and thus are displaced by faster-growing species that can take advantage of the additional nutrient supply. In the Netherlands, nitrogen deposition from upwind industrial and agricultural regions has caused species-rich plant communities to give way to species-poor communities, contributing measurably to a recent loss of plant species diversity there.


Go to MEDIA CLIP 45.1 Tracking Dead Zones from Space
PoL2e.com/mc45.1
924
Movement of carbon is linked to energy flow through ecosystems
All the macromolecules that make up living organisms contain carbon, and much of the energy that organisms use to fuel their metabolic activities comes from the oxidation of organic carbon compounds. The movement of carbon into, through, and out of communities is therefore intimately linked to energy flow through ecosystems, and biomass is an important compartment of the carbon cycle (FIGURE 45.10). Most of the carbon in the atmosphere occurs as the gases CO2 (carbon dioxide) and CH4 (methane), which are mixed on a global scale by the solar-powered circulation of Earth’s atmosphere (see Concept 41.2). These same forms are found dissolved in the oceans and in fresh water, and aquatic systems also harbor carbonate (CO32−). By far the largest pools of carbon occur in fossil fuels and in rocks containing carbonate compounds.


Go to ANIMATED TUTORIAL 45.3 The Global Carbon Cycle
PoL2e.com/at45.3
Fluxes of carbon are driven by biological, chemical, and physical processes. The biochemical process of photosynthesis moves carbon from inorganic compartments in the atmosphere and water into the organic compartment; cellular respiration reverses this flux. Carbon dioxide and methane move from their atmospheric compartments into the much larger compartments in the oceans when they dissolve, and they are returned to the atmosphere by outgassing; both of these movements are purely physical processes. The rate at which CO2 dissolves is slightly greater than the rate at which it is outgassed, for two reasons. First, some of the dissolved CO2 is converted into organic compounds by marine primary production. Most of this carbon is then recycled through the trophic interactions of aquatic organisms, but gravity moves a steady rain of nonliving organic matter into sediments in the benthic zone, where geological processes transform it eventually into fossil fuels such as coal, natural gas, and petroleum. Second, some of the dissolved CO2 is incorporated into relatively insoluble carbonate compounds, especially calcium carbonate. These also ultimately reach the sediments and form carbonate rocks such as limestone. The chemical reactions involved in carbonate formation are inorganic, but they are orchestrated by marine organisms when they form their shells (see Figure 20.10).
Human activities influence the carbon cycle in a number of ways. Anything that changes primary productivity, such as nitrogen deposition or altered land use, alters the movement of carbon between inorganic and organic compartments. Any activity that affects water runoff, such as deforestation or impoundment or alteration of river flows, affects the movement of carbon between the terrestrial and aquatic compartments. Burning of organic matter, whether biomass or fossil fuels, increases the atmospheric pool of CO2, as does the heating of calcium carbonate in the manufacture of cement. The atmospheric pool of methane (CH4) is increased by livestock production, rice cultivation, and water storage in reservoirs, because microbes in the guts of cattle and in waterlogged sediments break down organic compounds anaerobically to produce methane gas. Although the atmospheric pool of CH4 is far smaller than that of CO2, both are potent absorbers of infrared radiation and affect Earth’s radiation balance, as we will soon see.
925
APPLY THE CONCEPT: Certain biogeochemical cycles are especially critical for ecosystems
Figure 45.10 depicts the global carbon cycle, showing organic and inorganic pools of carbon in various compartments as well as fluxes. Refer to that figure to answer the following questions.
- What percentage of the flux of carbon from terrestrial ecosystems into the atmosphere is due to human activities?
- What other flux of carbon from terrestrial ecosystems is being influenced by humans, and how?
- Given the biogeochemical cycles we have discussed so far, which additional two cycles of materials would you wish to explore next in order to understand ecosystem function, and why? (Hint: Consider the major chemical constituents of living tissue, discussed in Concept 2.1.)
926
Biogeochemical cycles are not independent
The water, nitrogen, and carbon cycles illustrate the diversity of processes, relative sizes of pools and fluxes, and spatial and temporal scales that are involved in the cycling of materials within the Earth system. As you can imagine, there is even more variation if we consider other materials with different chemical and physical properties. For example, few forms of phosphorus are gases, and the phosphorus cycle lacks a significant atmospheric component. Similarly, sulfur released into the atmosphere by volcanic eruptions reacts with other gases to form tiny, airborne particles that affect Earth’s radiation balance (See Concept 45.4).
Go to ACTIVITY 45.1 The Phosphorus and Sulfur Cycles
PoL2e.com/at45.1
This diversity does not mean that different biogeochemical cycles are entirely independent of one another, however. The cycles of many materials are at least partly linked, for several reasons.
First, the fluxes of different materials are linked if those fluxes share common physical or chemical processes. For example, many elements, including carbon, nitrogen, phosphorus, and sulfur, have water-soluble forms, which are transported together in precipitation and runoff. If runoff increases, the fluxes of all water-soluble materials from terrestrial to aquatic compartments will increase.
Second, the fluxes of different materials through communities are linked because the functional macromolecules in living tissue—proteins, nucleic acids, polysaccharides, and so forth—have a precise structure with fixed proportions of elemental building blocks. Thus when biomass is produced, there is coordinated movement of these building blocks into living organic compartments, and when biomass is decomposed or burned, they all move back into inorganic compartments.
Fluxes can also interact in more subtle ways. Increased atmospheric concentrations of CO2, for example, can increase the water-use efficiency of many terrestrial plants. These plants obtain the CO2 they need for photosynthesis by opening their stomata, which also leads to transpiration—the loss of water vapor from their tissues. In a high-CO2 environment, these plants can leave their stomata open for a shorter time and therefore transpire less water, which slows the rate of water movement from soil to the atmosphere.
CHECKpoint CONCEPT 45.3
- How is the cycling of water important to the cycling of other materials?
- Why is nitrogen often a limiting nutrient for plant growth, even though it is the most abundant element in Earth’s atmosphere?
- Explain why the rate with which CO2 outgasses from oceans and fresh waters is lower than the rate with which it dissolves into those waters.
Biogeochemical cycles with significant atmospheric pools can be especially important for Earth’s climate, as we will see next.