1.2 CHEMICAL AND PHYSICAL PRINCIPLES

We stated earlier that biology is the study of life. But what exactly is life? As simple as this question seems, it is frustratingly difficult to answer. We all recognize life when we see it, but coming up with a definition is harder than it first appears.
Living organisms are clearly different from nonliving things. But just how different is an organism from the rock shown in Fig. 1.4? On one level, the comparison is easy: The rock is much simpler than any living organism we can think of. It has far fewer components, and it is largely static, with no apparent response to environmental change on time scales that are readily tracked.
In contrast, even an organism as relatively simple as a bacterium contains many hundreds of different chemical compounds organized in a complex manner. The bacterium is also dynamic in that it changes continuously, especially in response to the environment. Organisms reproduce, which minerals do not. And organisms do something else that rocks and minerals don’t: They evolve. Indeed, the molecular biologist Gerald Joyce has defined life as a chemical system capable of undergoing Darwinian evolution.
From these simple comparisons we can highlight four key characteristics of living organisms: (1) complexity, with precise spatial organization on several scales; (2) the ability to change in response to the environment; (3) the ability to reproduce; and (4) the capacity to evolve. Nevertheless, the living and nonliving worlds share an important attribute: Both are subject to the basic laws of chemistry and physics.
1.2.1 The living and nonliving worlds share the same chemical foundations and obey the same physical laws.
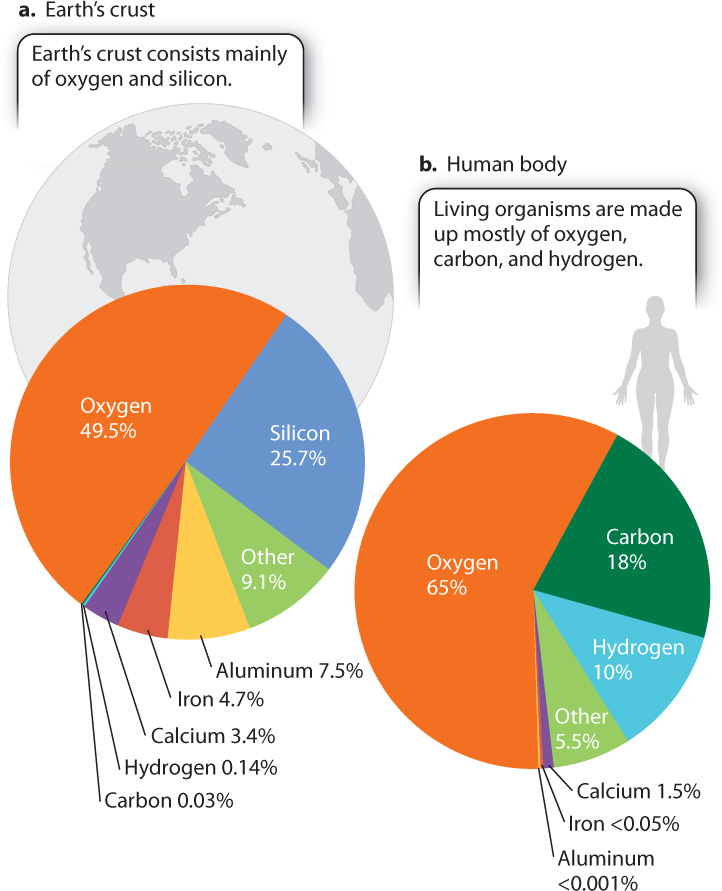
The chemical elements found in rocks and other nonliving things are no different from those found in living organisms. In other words, all the elements that make up living things can be found in the nonliving environment—there is nothing special about our chemical components when taken individually. That said, the relative abundances of elements in organisms differ greatly from those in the nonliving world. In the universe as a whole, hydrogen and helium make up more than 99% of known matter, while Earth’s crust contains mostly oxygen and silicon, with significant amounts of aluminum, iron, and calcium (Fig. 1.5a). In organisms, by contrast, oxygen, carbon, and hydrogen are by far the most abundant elements (Fig. 1.5b). As discussed more fully in Chapter 2, carbon provides the backbone of life’s chemistry. The particular properties of carbon make possible a wide diversity of molecules that, in turn, support a wide range of functions and activities within cells.
All living organisms are subject to the physical laws of the universe. Physics helps us to understand how animals move and why trees don’t fall over; it explains how redwoods conduct water upward through their trunks and how oxygen gets into the cells that line your lungs. Indeed, two laws of thermodynamics, both of which describe how energy is transformed in any system, determine how living organisms are able to do work and maintain their spatial organization.
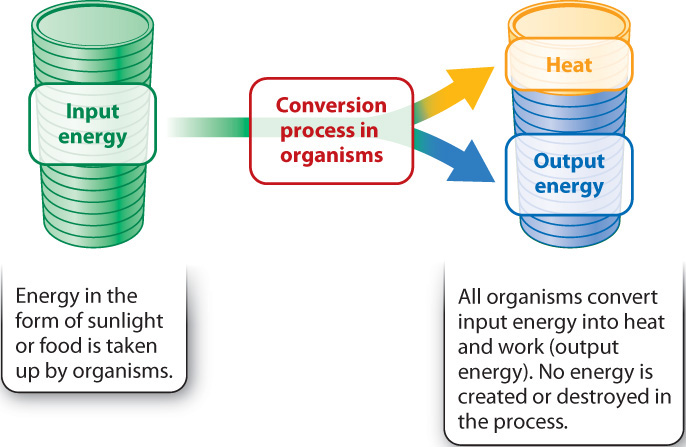
The first law of thermodynamics states that energy can neither be created nor destroyed; it can only be transformed from one form into another. In other words, the total energy in the universe is constant, but the form that energy takes can change. Living organisms are energy transformers. They acquire energy from the environment and transform it into a chemical form that cells can use. All organisms obtain energy from the sun or from chemical compounds. As they harness this energy, some is used to do work—such as moving, reproducing, and building cellular components—and the rest is dissipated as heat. The energy that is used to do work plus the heat that is generated is the total amount of energy, which is the same as the input energy (Fig. 1.6). In other words, the total amount of energy remains constant.
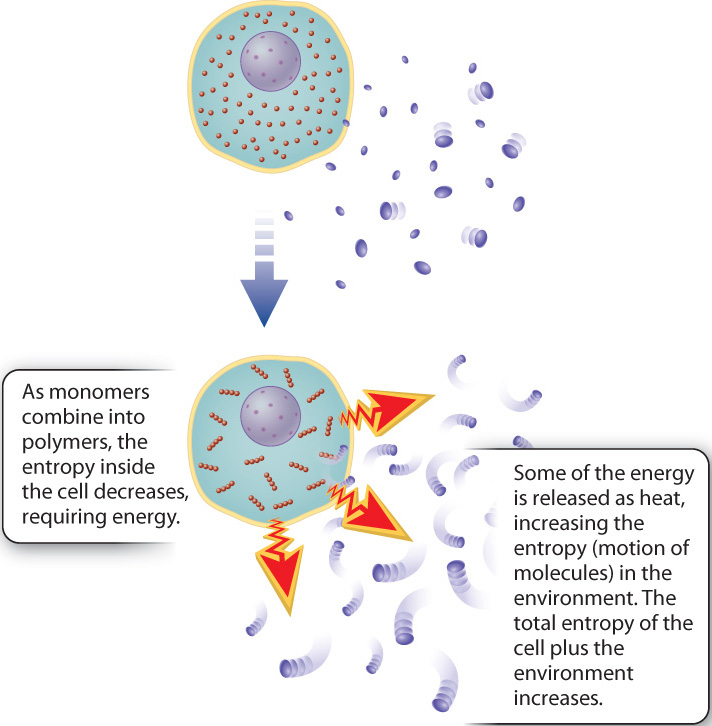
The second law of thermodynamics states that the degree of disorder in the universe tends to increase. To understand order and disorder in this context, think about a box full of marbles distributed more or less randomly; if you want to line up all the red ones or blue ones in a row, you have to do work. That is, you have to add energy. In this case, the addition of energy increases the order of the system, or, put another way, decreases its disorder. Physicists quantify the amount of disorder in a system, describing it as the entropy of the system.
Living organisms are highly organized. As with lining up marbles in a row, energy is needed to maintain this organization. Given the tendency toward greater disorder, the high level of organization of even a single cell would appear to violate the second law. But it does not. The key is that a cell is not an isolated system and therefore cannot be considered on its own; it exists in an environment. So we need to take into account the whole system, the cell plus the surrounding environment. As energy is harnessed by cells, only some is used to do work; the rest is dissipated as heat (Fig. 1.6). That is, conversions of energy from one form to another are never 100% efficient. Heat is a form of energy, so the total amount of energy is conserved, as dictated by the first law. In addition, heat corresponds to the motion of small molecules—the greater the heat, the greater the motion, and the greater the disorder. Therefore, the release of heat as organisms harness energy means that the total entropy for the combination of the cell and its surroundings increases, in keeping with the second law (Fig. 1.7).
1.2.2 The scientific method shows that living organisms come from other living organisms.
Life is made up of chemical components that also occur in the nonliving environment and obey the same laws of chemistry and physics. Can life spontaneously arise from these nonliving materials? We all know that living organisms come from other living organisms, but it is worth asking how we know this. Direct observation can be misleading here. For example, raw meat, if left out on a plate, will rot and become infested with maggots (fly larvae). It might seem as though the maggots appear spontaneously. In fact, the question of where maggots come from was a matter of vigorous debate for centuries, until application of the scientific method settled the issue. In the 1600s, the Italian physician and naturalist Francesco Redi hypothesized that maggots (and hence flies) in rotting meat come only from other flies that laid their eggs in the meat.
To test his hypothesis, Redi set up an experiment in which he placed meat in three glass jars (Fig. 1.8). One jar was left open, a second was covered with gauze, and the third was sealed with a cap. The jars were left in a room with flies. Note that in this experiment, the three jars were subject to the same conditions—the only difference was the opening of the jar. The open jar allowed for the passage of flies and air; the jar with the gauze allowed for the passage of air but not flies; and the sealed jar did not allow air or flies to enter. Over time, Redi observed that maggots appeared only on the meat in the open jar. No maggots appeared in the other two jars, which did not allow access to the meat by flies. These observations supported Redi’s hypothesis that flies come from other flies, and did not provide support for the alternative hypothesis that maggots and flies arise spontaneously from meat.
FIG. 1.8Can living organisms arise from nonliving matter?
BACKGROUND Until the 1600s, many people believed that rotting meat spontaneously generates maggots (fly larvae).
HYPOTHESIS Francesco Redi hypothesized that maggots come only from flies and are not spontaneously generated.
EXPERIMENT Redi used three jars containing meat. One jar was left open; one was covered with gauze; one was sealed with a cap.
RESULTS
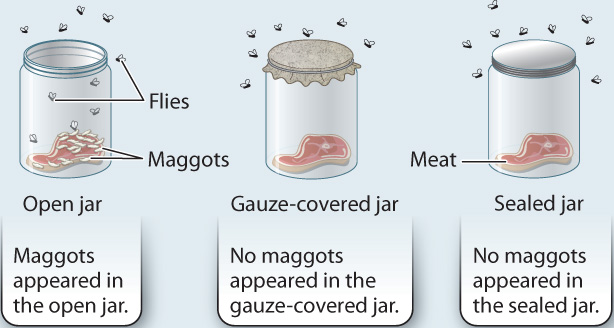
CONCLUSION The presence of maggots in the open jar and the absence of maggots in the gauze-covered and sealed jars supported the hypothesis that maggots come from flies, and allowed Redi to reject the hypothesis that maggots are spontaneously generated.
FOLLOW-UP WORK Redi’s experiment argued against the idea of spontaneous generation for insects. However, it was unclear whether his results could be extended to microbes. Applying the scientific method, Louis Pasteur used a similar approach about 200 years later to investigate this question (see Fig. 1.9).
Redi demonstrated that living organisms come from other organisms, but some argued that his conclusion might apply only to larger organisms—microscopic life might be another matter entirely. It was not until the nineteenth century that the French chemist and biologist Louis Pasteur tested the hypothesis that microorganisms can arise by spontaneous generation (Fig. 1.9).
FIG. 1.9Can microscopic life arise from nonliving matter?
BACKGROUND Educated people in Pasteur’s time knew that microbes grow well in nutrient-rich liquids like broth. It was also known that boiling would sterilize the broth, killing the microbes.
HYPOTHESIS Pasteur hypothesized that if microbes were generated spontaneously from nonliving matter, they should reappear in sterilized broth without the addition of microbes.
EXPERIMENT Pasteur used two flasks, one with a straight neck and one with a swan neck. The straight-neck flask allowed dust particles with microbes to enter. The swan-neck flask did not.
RESULTS
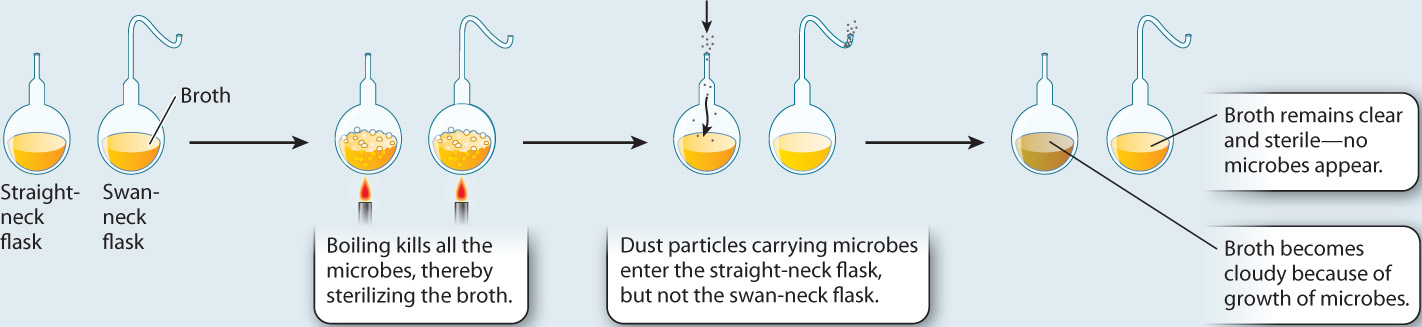
CONCLUSION The presence of microbes in the straight-neck flask and the absence of microbes in the swan-neck flask supported the hypothesis that microbes come from other microbes and are not spontaneously generated.
DISCUSSION Redi’s and Pasteur’s research illustrate classic attributes of well-designed experiments. Multiple treatments are set up, and nearly all conditions are the same in them all—they are constant, and therefore cannot be the cause of different outcomes of the experiment. One key feature—the variable—is changed by the experimenter from one treatment to the next. This is a place to look for explanations of different experimental outcomes.
Pasteur filled two glass flasks with heat-sterilized broth—one with a straight vertical neck and the other with a curved swan neck. As in Redi’s experiments, there was only one variable, in this case the shape of the neck of the flask. The straight-neck flask allowed airborne dust particles carrying microbes to fall into the sterile broth, while the swan-neck flask prevented dust from getting inside. Over time, Pasteur observed that microbes grew in the broth inside the straight-neck flask but not in the swan-neck flask. From these observations, Pasteur rejected the hypothesis that microbes arise spontaneously from sterile broth. Instead, exposure to microbes carried on airborne dust particles is necessary for microbial growth.
Redi’s and Pasteur’s experiments demonstrated that living organisms come from other living organisms and are not generated spontaneously from chemical components. But this raises the question of how life arose in the first place. If life comes from life, where did the first living organisms come from? Although today all organisms are produced by parental organisms, early in Earth’s history this was probably not the case. Scientists hypothesize that life initially emerged from chemical compounds about 4 billion years ago. That is, chemical systems capable of evolution arose from chemical reactions that took place on the early Earth. We’ll return to the great question of the origin of life in Case 1: The First Cell and in Chapters 2 through 8.