2.3 WATER: THE MEDIUM OF LIFE
On Earth, all life depends on water. Indeed, life originated in water, and the availability of water strongly influences the environmental distributions of different species. Furthermore, water is the single most abundant molecule in all cells, so water is the medium in which the molecules of life interact. In the late 1990s, the National Aeronautic and Space Administration (NASA) announced that the search for extraterrestrial life would guide continuing exploration of the solar system and beyond. NASA’s operational strategy was simple: Follow the water. NASA’s logic was straightforward: Within our solar system, Earth stands out both for its abundance of water and the life it supports. What makes water so special as the medium of life?
2.3.1 Water is a polar molecule.
As we saw earlier, water molecules have polar covalent bonds, characterized by an uneven distribution of electrons. A molecule like water that has regions of positive and negative charge is called a polar molecule. Molecules, or even different regions of the same molecule, fall into two general classes, depending on how they interact with water: hydrophilic (“water loving”) and hydrophobic (“water fearing”).
Hydrophilic compounds, like water itself, are polar; they dissolve readily in water. That is, water is a good solvent, capable of dissolving many substances. Think of what happens when you stir a teaspoon of sugar into water: The sugar seems to disappear as the sugar dissolves. What is happening is that the sugar molecules are dispersing through the water and becoming separated from one another. Sugar is in solution in the watery, or aqueous, environment.
By contrast, hydrophobic compounds are nonpolar and arrange themselves to minimize their contact with water. For example, when oil and water are mixed, oil molecules organize themselves into droplets that limit the oil–water interface. This hydrophobic effect, in which polar molecules like water exclude nonpolar ones, drives such biological processes as the formation of cell membranes (Chapter 3) and the folding of proteins (Chapter 5).
2.3.2 pH is a measure of the concentration of protons in solution.
A small proportion of the molecules in water exist as protons (H+) and hydroxide ions (OH−). The pH of a solution measures the proton concentration ([H+]) by the following formula:
pH = −log [H+]
The pH of a solution can range from 0 to 14. Since the pH scale is logarithmic, a difference of one pH unit corresponds to a tenfold difference in hydrogen ion concentration. A solution is neutral (pH = 7) when the concentrations of protons (H+) and hydroxide ions (OH−) are equal. When the concentration of protons is higher than that of hydroxide ions, then the pH is lower than 7 and the solution is acidic. When the concentration of protons is lower than that of hydroxide ions, then the pH is higher than 7, and the solution is basic. An acid therefore is a molecule that releases a proton (H+), and a base is a molecule that accepts a proton in aqueous solution.
Pure water has a pH of 7—that is, it is neutral, with an equal concentration of protons and hydroxide ions. The pH of most cells is approximately 7 and is tightly regulated, as most chemical reactions can be carried out only in a narrow pH range. Certain cellular compartments, however, have a much lower pH. The pH of blood is slightly basic, with a pH around 7.4. This value is sometimes referred to in medicine as physiological pH, as it can change in response to certain diseases. Freshwater lakes, ponds, and rivers tend to be slightly acidic because of dissolved carbon dioxide from the air, which forms carbonic acid in water.
2.3.3 Hydrogen bonds give water many unusual properties.
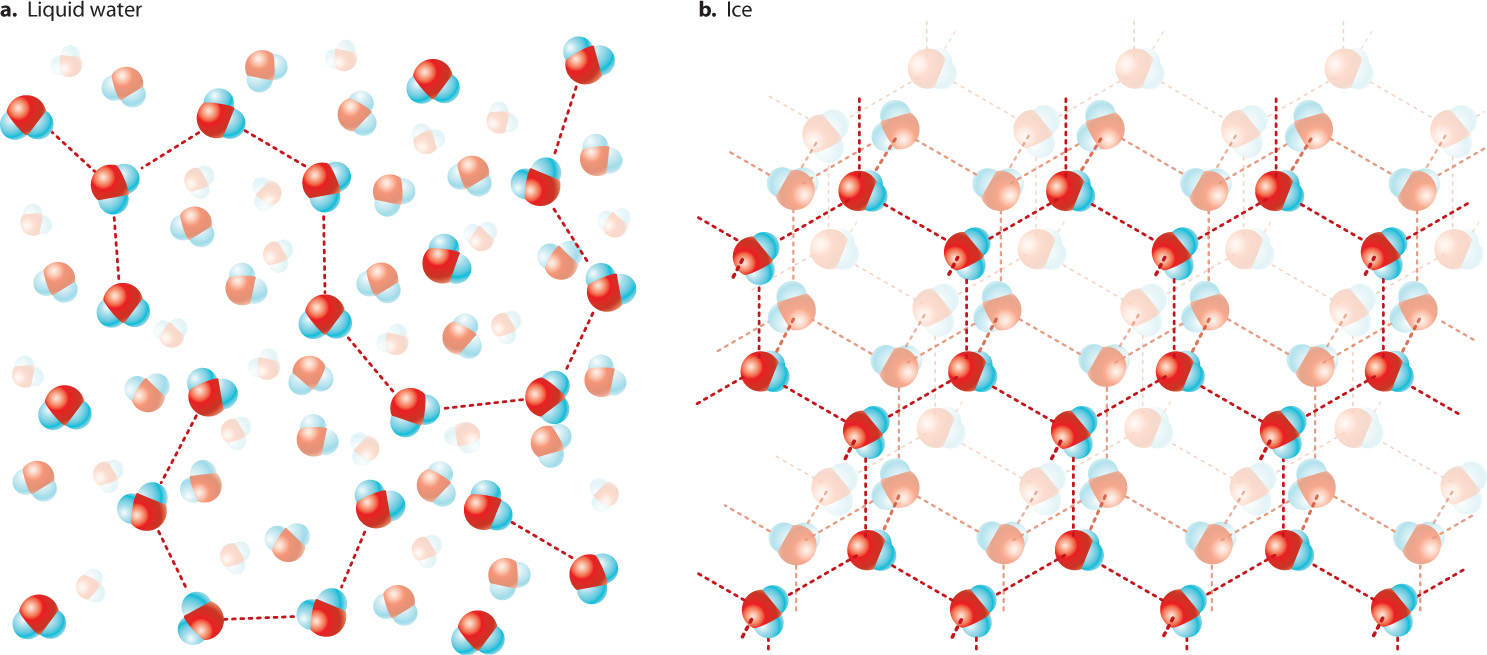
Water is also characterized by extensive hydrogen bonding, as we have seen. Hydrogen bonds influence the structure of both liquid water (Fig. 2.11a) and ice (Fig. 2.11b). When water freezes, most water molecules become hydrogen bonded to four other water molecules, forming an open lattice-like, crystalline structure we call ice. As the temperature increases and the ice melts, some of the hydrogen bonds are destabilized. This allows the water molecules to pack more closely, and it is the reason why liquid water is more dense than solid water. As a result, ice floats on water, and ponds and lakes freeze from the top down, and therefore do not freeze completely. This special property allows fish and aquatic plants to survive winter in the cold water under the layer of ice.
Quick Check 3
Why do containers of water, milk, soda, or other liquids sometimes burst when frozen?
Hydrogen bonds also make water molecules cohesive, meaning that they tend to stick to one another. A consequence of cohesion is high surface tension, a measure of the difficulty of breaking the surface of a liquid. Water cohesion and surface tension contribute to water movement in plants. As water evaporates from leaves, water is pulled upward, sometimes as high as 300 feet above the ground in giant sequoia and coast redwood trees, which are among the tallest trees on Earth.
The hydrogen bonds of water also influence how water responds to heating. Molecules are in constant motion, and this motion increases as the temperature increases. When water is heated, however, the increased motion first breaks hydrogen bonds, and only afterward leads to a temperature increase. The need to break hydrogen bonds first means that water resists temperature changes more than do other substances, a property that is important for living organisms on a variety of scales. In the cell, water resists temperature variations that would otherwise result from numerous biochemical reactions. On a global scale, the oceans minimize temperature fluctuations, stabilizing the temperature on Earth in a range compatible with life.
In short, water is clearly the medium of life on Earth, but is this because water is uniquely suited for life, or is it because life on Earth has adapted through time to a watery environment? We don’t know the answer, but probably both explanations are partly true. Chemists have proposed that under conditions of high pressure and temperature, other small molecules, among them ammonia (NH3) and some simple carbon-containing molecules, might display similar characteristics friendly to life. However, under the conditions of pressure and temperature that exist on Earth, water is the only molecule uniquely suited to life. Water is a truly remarkable substance, and life on Earth would not be possible without it. Leonardo da Vinci once wrote that water is the driving force of all nature. Without a doubt, water is the driving force of all biology.