2.1 PROPERTIES OF ATOMS
Teaching Tip:
This is just some interesting factoid. Wow, who would have guessed?
Since antiquity, it has been accepted that the materials of nature are made up of a small number of fundamental substances combined in various ways. Aristotle called these substances elements, and recognized four: earth, air, fire, and water. From the seventeenth century through the end of the nineteenth, elements were defined as pure substances that could not be broken down further by the methods of chemistry. In time, it was recognized that each element contains only one type of atom, the basic unit of matter. By 1850, about 60 elements were known, including such common ones as oxygen, copper, gold, and sodium. Today, 118 elements are known, of which 94 occur naturally and 24 have been created artificially in the laboratory. Elements are often indicated by a chemical symbol, which consists of a one-or two-letter abbreviation of the name of the element. For example, carbon is represented by C, hydrogen by H, and helium by He.
2.1.1 Atoms consist of protons, neutrons, and electrons.
Did you know...
This is just some interesting factoid. Wow, who would have guessed?
Elements are composed of atoms. The atom contains a dense central nucleus made up of positively charged particles called protons and electrically neutral particles called neutrons. A third type of particle, the negatively charged electron, moves around the nucleus at some distance from it. Carbon, for example, typically has six protons, six neutrons, and six electrons (Fig. 2.1).
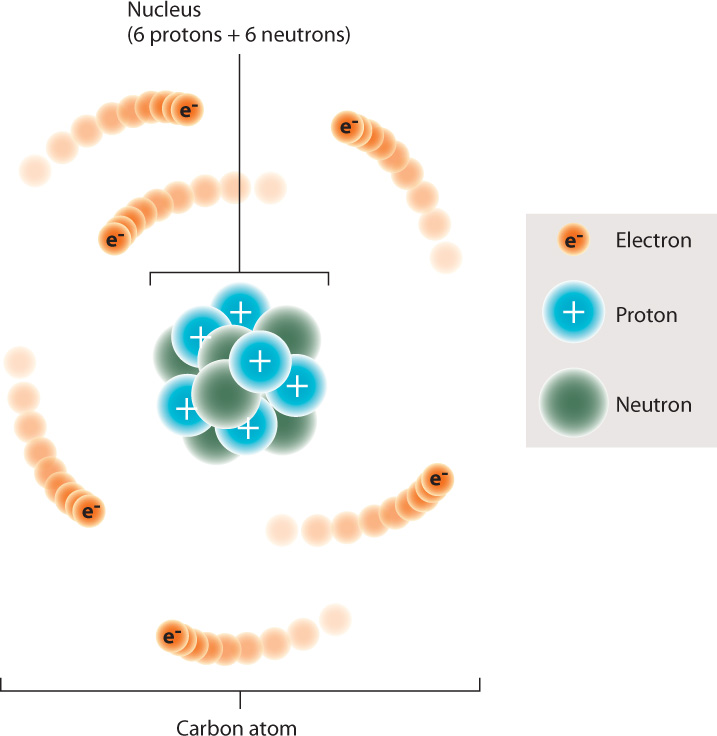
The number of protons, or the atomic number, specifies the atom as a particular element; an atom with one proton is hydrogen, for example, and an atom with six protons is carbon. The atomic number is sometimes indicated as a left subscript to the chemical symbol, for example 6C. Here, the “6” represents the number of protons in a carbon atom.
Did you know...
This is just some interesting factoid. Wow, who would have guessed?
More Info...
This is just some interesting factoid. Wow, who would have guessed?
There can be all kinds of stuff inside here.
Together, the protons and neutrons determine the atomic mass, the mass of the atom. Each proton and neutron, by definition, has a mass of 1, whereas an electron has negligible mass. The number of neutrons in atoms of a particular element can vary, changing its mass. Isotopes are atoms of the same element that have different numbers of neutrons. For example, carbon has three isotopes: About 99% of carbon atoms have six neutrons and six protons, for an atomic mass of 12; about 1% has seven neutrons and six protons, for an atomic mass of 13; and only a very small fraction has eight neutrons and six protons, for an atomic mass of 14. The atomic mass is sometimes indicated as a left superscript to the chemical symbol. For instance, 12C is the isotope of carbon with six neutrons and six protons.
Typically, an atom has the same number of protons and electrons. Because a carbon atom has six protons and six electrons, the positive and negative charges cancel each other out and the carbon atom is electrically neutral. Some chemical processes cause an atom to either gain or lose electrons. An atom that has lost an electron is positively charged, and one that has gained an electron is negatively charged. Electrically charged atoms are called ions. The charge of an ion is specified in the right superscript position next to the chemical symbol. Thus, H+ indicates a hydrogen ion that has lost an electron and is positively charged.
2.1.2 Electrons occupy regions of space called orbitals.
Electrons move around the nucleus, but not in the simplified way shown in Fig. 2.1. The exact path that an electron takes is unknown, but it is possible to identify a region in space, called an orbital, where an electron is present most of the time. For example, Fig. 2.2a shows the orbital for hydrogen, which is simply a sphere occupied by a single electron. Most of the time, the electron is found within the space defined by the sphere, although its exact location at any instant is unpredictable.
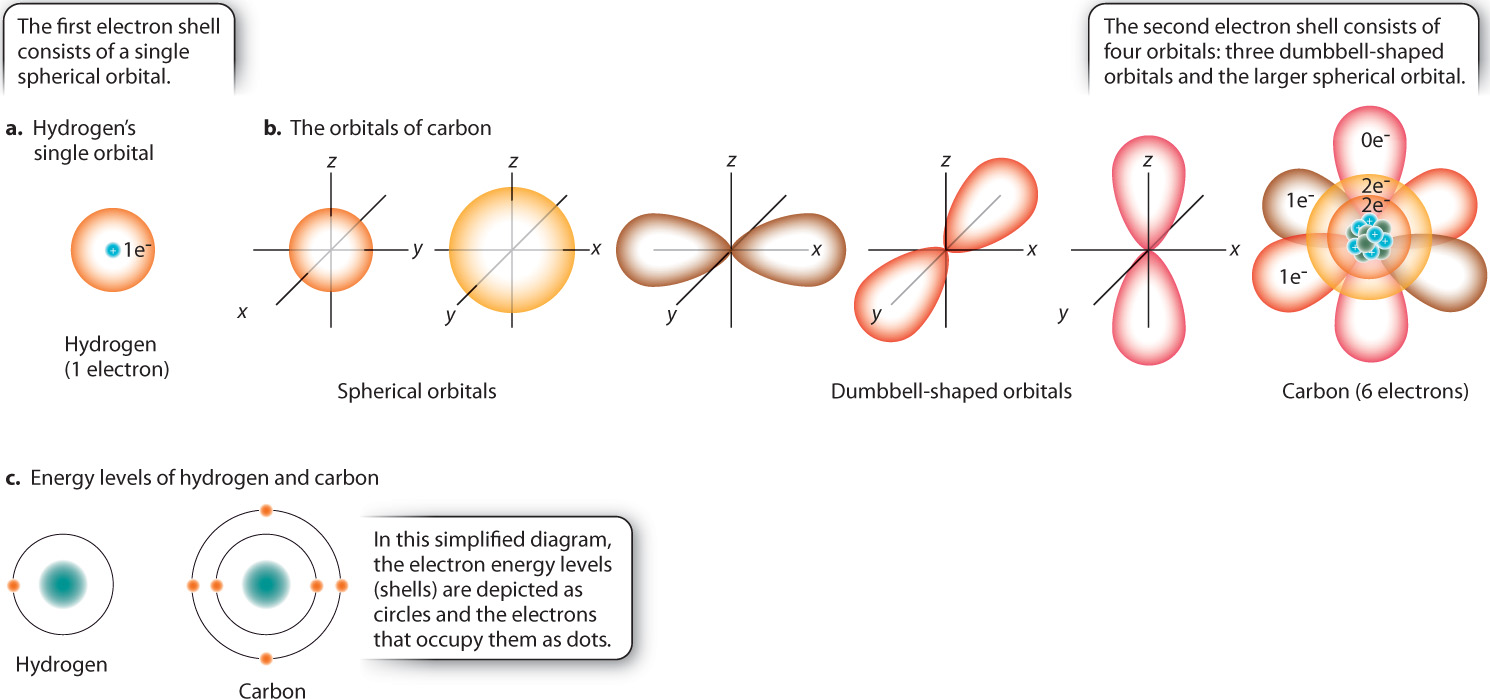
Orbitals have certain properties. The maximum number of electrons in any orbital is two. Most atoms have more than two electrons and so have several orbitals positioned at different distances from the nucleus. These orbitals differ in size and shape. Electrons in orbitals close to the nucleus have less energy than do electrons in orbitals farther away, so electrons fill up orbitals close to the nucleus before occupying those farther away. Several orbitals can exist at a given energy level, or shell. The first shell consists of the spherical orbital shown in Fig. 2.2a.
Fig. 2.2b shows electron orbitals for carbon. Of carbon’s six electrons, two occupy the small spherical orbital representing the lowest energy level. The remaining four are distributed among four possible orbitals at the next highest energy level: One of these four orbitals is a sphere (larger in diameter than that at the lowest energy level) and three are dumbbell-shaped. In carbon, the outermost spherical orbital has two electrons, two of the dumbbell-shaped orbitals have one electron each, and one of the dumbbell-shaped orbitals is empty. Because a full orbital contains two electrons, it would take a total of four additional electrons to completely fill all of the orbitals at this energy level. Therefore, after the first shell, the maximum number of electrons per energy level is eight. Fig. 2.2c shows that the highest energy level, or shell, of carbon, represented by the outermost circle, contains four electrons, and that of hydrogen contains one electron.
Quick Check 1
In the early 1900s, Ernest Rutherford produced a beam of very small negative particles and directed it at a thin piece of gold foil just a few atoms thick. Most of the particles passed through the foil without changing their path; very rarely, a particle was deflected. What conclusions can you draw from this experiment about the structure of an atom?
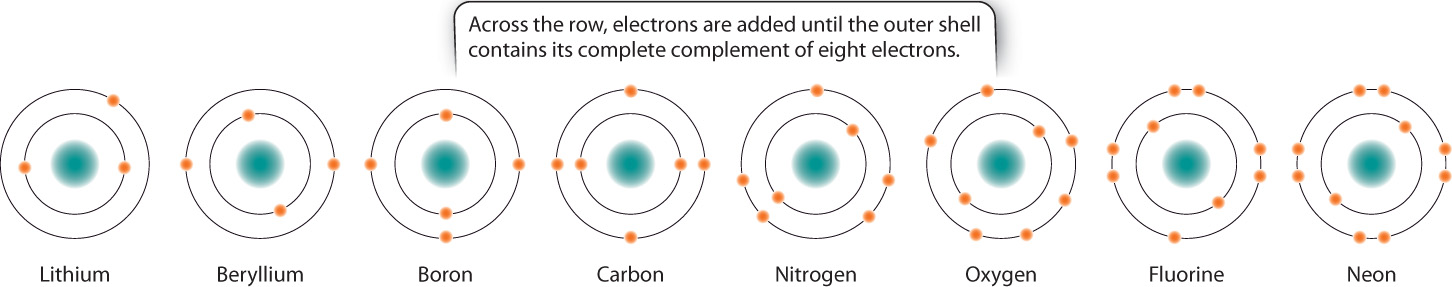
2.1.3 Elements have recurring, or periodic, chemical properties.
The chemical elements are often arranged in a tabular form known as the periodic table of the elements, shown in Fig. 2.3 and generally credited to the nineteenth-century Russian chemist Dmitri Mendeleev. The table provides a way to organize all the chemical elements in terms of their chemical properties.
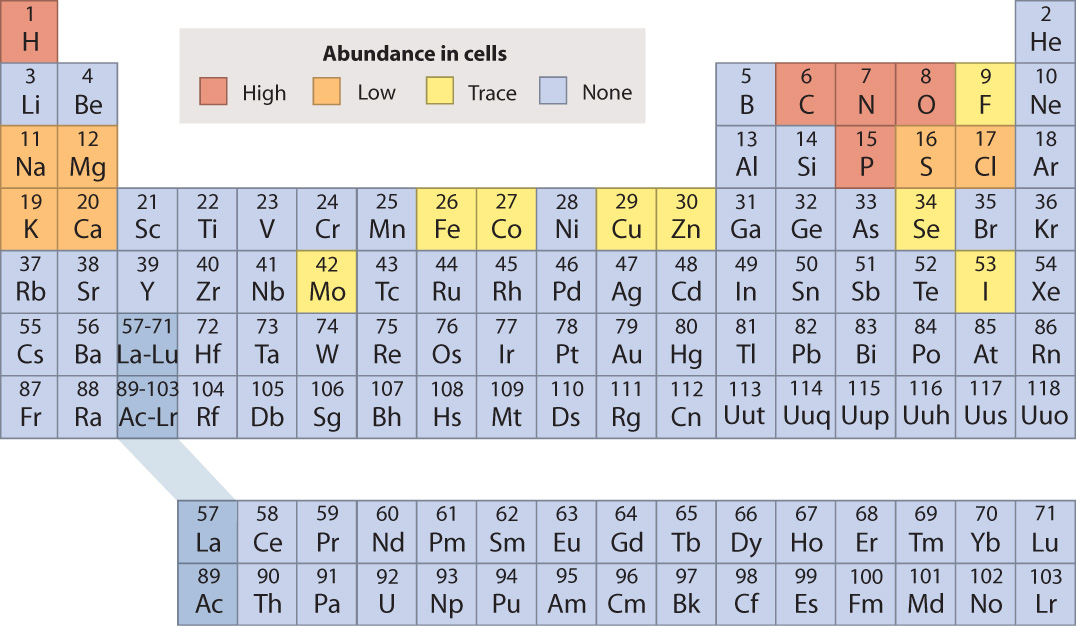
In the periodic table, the elements are indicated by their chemical symbols and arranged in order of increasing atomic number. For example, the second row of the periodic table begins with lithium (Li) with 3 protons and ends with neon (Ne) with 10 protons.
For the first three horizontal rows in the periodic table, elements in the same row have the same number and types of orbitals. Across a row, therefore, electrons fill the shell until a full complement of electrons is reached on the right-hand side of the table. Fig. 2.4 shows the filling of the shells for the second row of the periodic table. The elements in a vertical column are called a group or family. Members of a group all have the same number of electrons in their outermost orbital. For example, carbon (C) and lead (Pb) both have four electrons in their outermost orbital. The number of electrons in the outermost orbital determines in large part how elements behave and interact with other elements, as we will see in the next section.