2.2 MOLECULES AND CHEMICAL BONDS
Atoms can combine with other atoms to form molecules, which are substances made up of two or more atoms. When two atoms form a molecule, the individual atoms interact in what is called a chemical bond, a form of attraction between atoms that holds them together. The ability of atoms to form molecules in part explains why just a few types of element can make many different molecules and perform diverse functions in a cell. There are many different ways in which atoms can interact with one another, and therefore many different types of chemical bond, as we describe in this section.
2.2.1 A covalent bond results when two atoms share electrons.
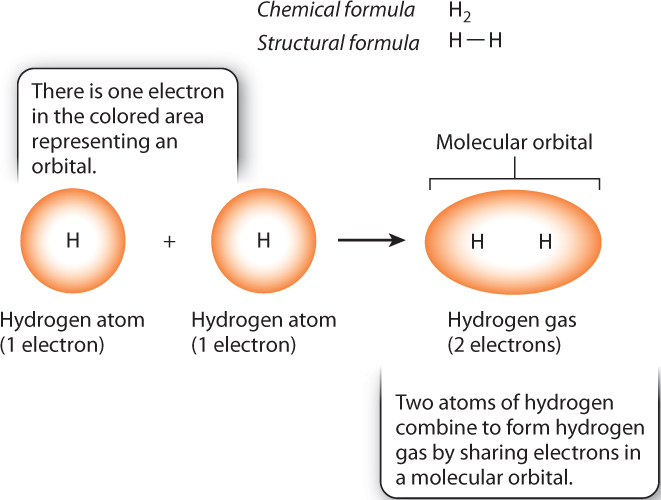
The ability of atoms to combine with other atoms is determined in large part by the electrons farthest from the nucleus—those in the outermost orbitals of an atom. These electrons are called valence electrons, and they are at the highest energy level. In many cases, when atoms combine with other atoms to form a molecule, the atoms share valence electrons with each other. Specifically, when the outermost orbitals of two atoms come into proximity, two atomic orbitals each containing one electron merge into a single orbital containing a full complement of two electrons. The merged orbital is called a molecular orbital, and each shared pair of electrons constitutes a covalent bond that holds the atoms together.
Hydrogen gas (H2), illustrated in Fig. 2.5, is one of the simplest molecules. Note that the right subscript position is used to indicate the number of atoms in a molecule. Each hydrogen atom has a single electron in a spherical orbital. When the atoms join into a molecule, the two orbitals merge into a single molecular orbital containing two electrons that are shared by the hydrogen atoms. A covalent bond between atoms is denoted by a single line connecting the two chemical symbols for the atoms, as shown in the structural formula in Fig. 2.5.
Quick Check 2
From their positions in the periodic table (see Fig. 2.3), can you predict how many lithium atoms and hydrogen atoms combine to form a molecule?
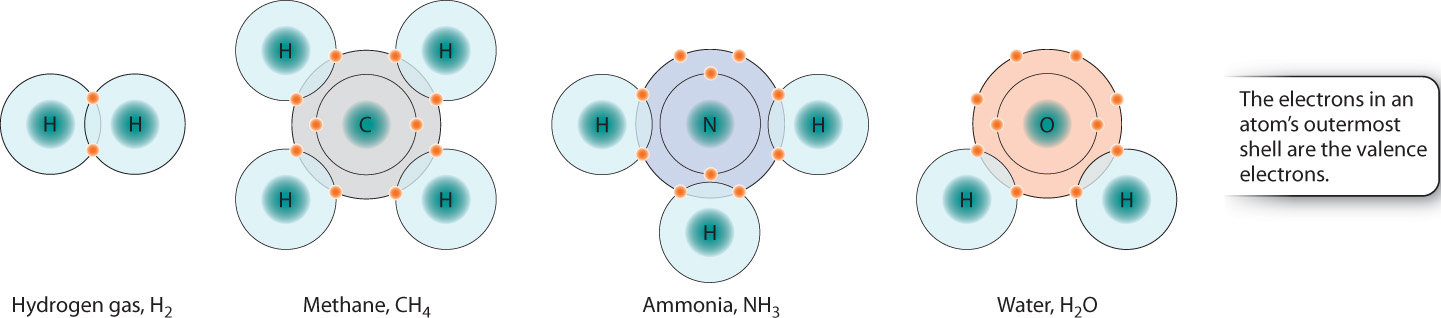
Molecules tend to be most stable when they share enough electrons to completely occupy the outermost energy level or shell. This simple rule of thumb is known as the octet rule and applies to many, but not all, elements. For example, as shown in Fig. 2.6, one carbon atom (C, with four valence electrons) combines with four hydrogen atoms (H, with one valence electron each) to form CH4 (methane); nitrogen (N, with five valence electrons) combines with three H atoms to form NH3 (ammonia); and oxygen (O, with six valence electrons) combines with two H atoms to form H2O (water). Interestingly, the elements of the same column in the next row behave similarly. This is just one example of the recurring, or periodic, behavior of the elements.
2.2.2 A polar covalent bond is characterized by unequal sharing of electrons.
In hydrogen gas, the electrons are shared equally by the two hydrogen atoms. In many bonds, however, the electrons are not shared equally by the two atoms. A notable example is provided by the bonds in a water molecule (H2O), which consists of two hydrogen atoms each covalently bound to a single oxygen atom (Fig. 2.7a).
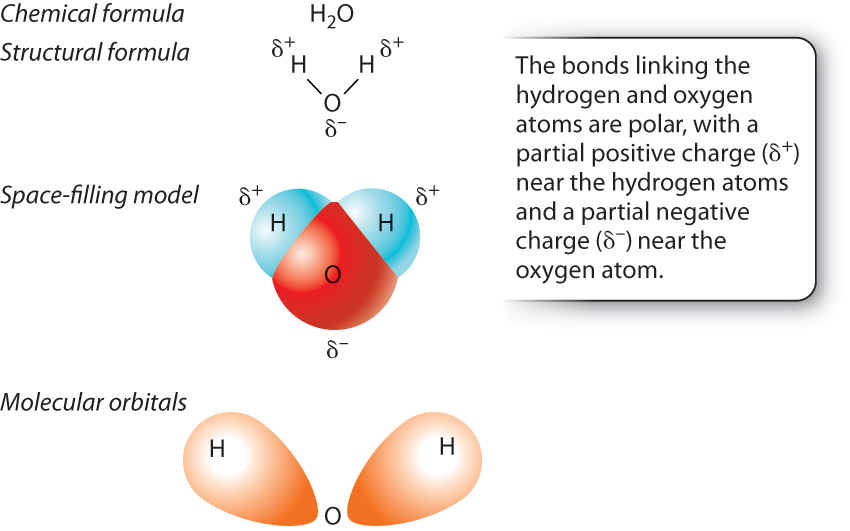
In a molecule of water, the electrons are not shared equally between the hydrogen and oxygen atoms; rather, the electrons are more likely to be located near the oxygen atom. Unequal sharing of electrons results from a difference in the ability of the atoms to attract electrons, a property known as electronegativity. Electronegativity tends to increase across a row in the periodic table; as the number of protons across a row increases, electrons are held more tightly to the nucleus. Therefore, oxygen is more electronegative than hydrogen and attracts electrons more than does hydrogen. In a molecule of water, oxygen has a slight negative charge, while the two hydrogen atoms have a slight positive charge (Fig. 2.7b). When electrons are shared unequally between the two atoms, the resulting interaction is described as a polar covalent bond.
2.2.3 A hydrogen bond is an interaction of a hydrogen atom and an electronegative atom.
Because the oxygen and hydrogen atoms have slight charges, water molecules orient themselves to minimize the repulsion of like charges so that positive charges are near negative charges. A hydrogen bond results when a hydrogen atom covalently bound to an electronegative atom (such as oxygen or nitrogen) interacts with an electronegative atom of another molecule. In the case of water, a hydrogen atom covalently bound to an oxygen atom is attracted to and interacts with an oxygen atom of another water molecule. The result of many such interactions is a kind of molecular network stabilized by hydrogen bonds. Typically, a hydrogen bond is depicted by a dotted line, as in Fig. 2.8.
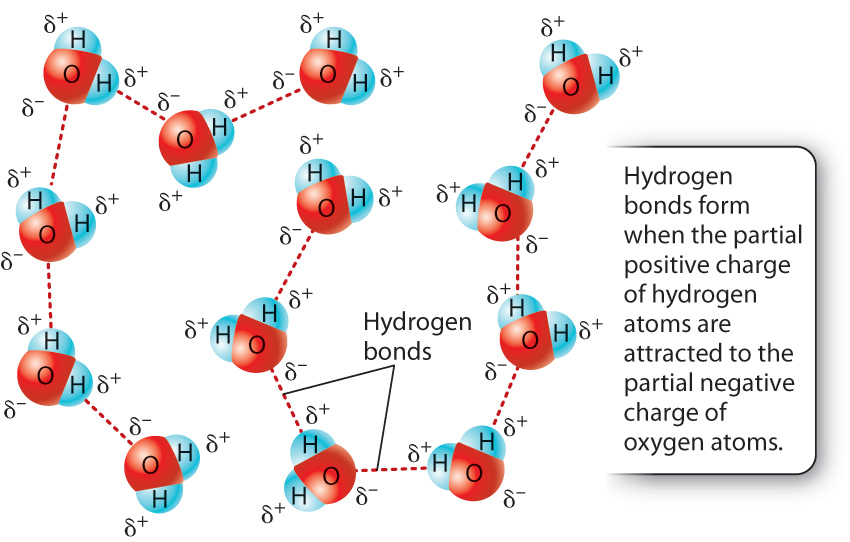
Hydrogen bonds are much weaker than covalent bonds, but it is hydrogen bonding that gives water many of its unusual properties, which are described in the next section. In addition, many weak hydrogen bonds can help stabilize biological molecules, as in the case of nucleic acids and proteins.
2.2.4 An ionic bond forms between oppositely charged ions.
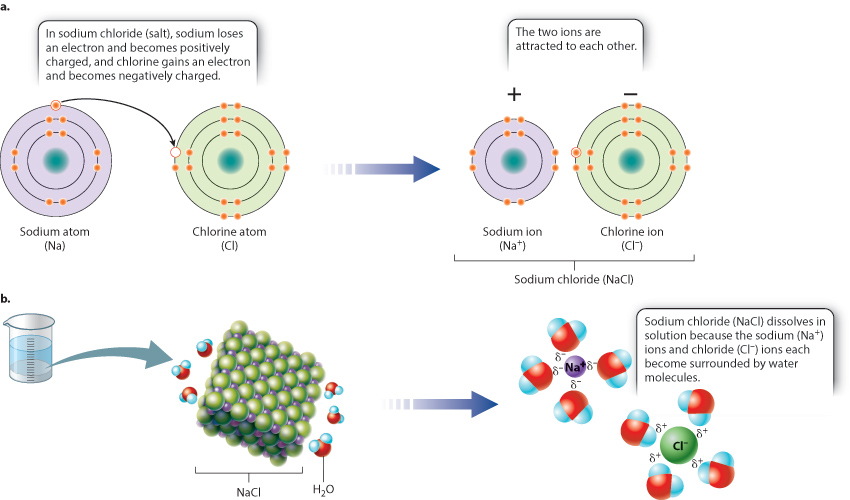
In water, the difference in electronegativity between the oxygen and the hydrogen atoms leads to unequal sharing of electrons. In more extreme cases, when an atom of very high electronegativity is paired with an atom of very low electronegativity, the difference in electronegativity is so great that the electronegative atom “steals” the electron from its less electronegative partner. In this case, the atom with the extra electron has a negative charge and is a negative ion. The atom that has lost an electron has a positive charge and is a positive ion. The two ions are not covalently bound, but they associate with each other because of the attraction of opposite charges in what is called an ionic bond. An example of a compound formed by the attraction of a positive ion and a negative ion is table salt, or sodium chloride (NaCl) (Fig. 2.9a).
When sodium chloride is placed in water, the salt dissolves to form sodium ions (written as “Na+”) that have lost an electron and so are positively charged, and chloride ions (Cl−) that have gained an electron and are negatively charged. In solution, the two ions are pulled apart and become surrounded by water molecules: The negatively charged ends of water molecules are attracted to the positively charged sodium ion and the positively charged ends of other water molecules are attracted to the negatively charged chloride ion (Fig. 2.9b). Only as the water evaporates do the concentrations of Na+ and Cl− increase to the point where the ions join and precipitate as salt crystals.
2.2.5 A chemical reaction involves breaking and forming chemical bonds.
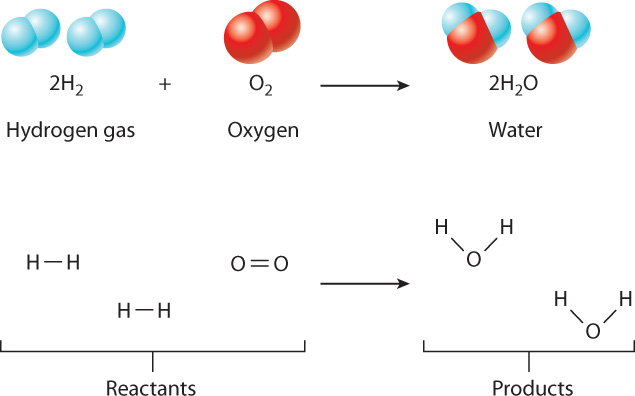
The chemical bonds that link atoms in molecules can change in a chemical reaction, a process by which given molecules, called reactants, are transformed into different molecules, called products. During a chemical reaction, atoms keep their identity but change their chemical bonds.
For example, two molecules of hydrogen gas (2H2) and one molecule of oxygen (O2) can react to form two molecules of water (2H2O), as shown in Fig. 2.10. In this reaction, the numbers of each type of atom are conserved, but their arrangement is different in the reactants and the products. Specifically, the H–H bond in hydrogen gas and the O O bond in oxygen are broken. At the same time, each oxygen atom forms new covalent bonds with two hydrogen atoms, forming two molecules of water. In fact, this reaction is the origin of the name “hydrogen,” which literally means “water former.” The reaction releases a good deal of energy; it was used in the main engine of the Space Shuttle.
O bond in oxygen are broken. At the same time, each oxygen atom forms new covalent bonds with two hydrogen atoms, forming two molecules of water. In fact, this reaction is the origin of the name “hydrogen,” which literally means “water former.” The reaction releases a good deal of energy; it was used in the main engine of the Space Shuttle.
In biological systems, chemical reactions provide a way to build and break down molecules for use by the cell, as well as to harness energy, which can be stored in chemical bonds (Chapter 6).