2.4 CARBON: LIFE’S CHEMICAL BACKBONE
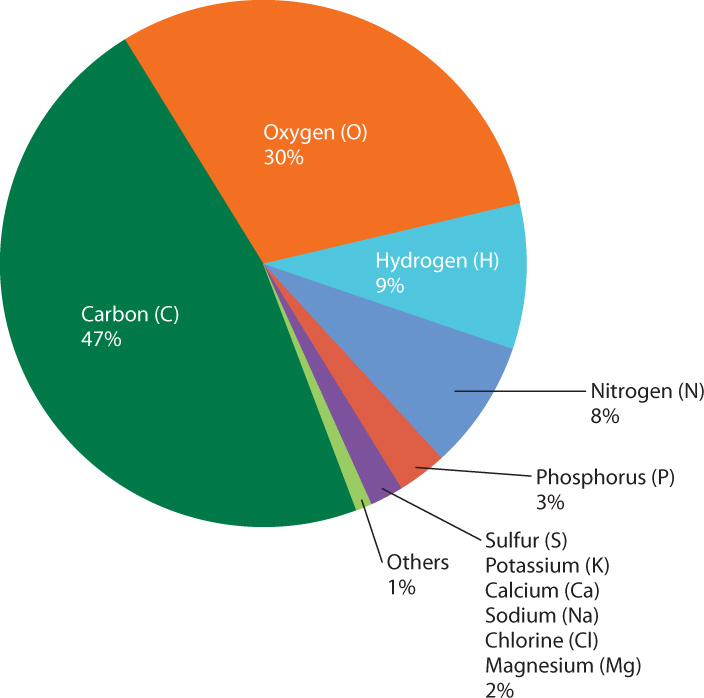
Hydrogen and helium are far and away the most abundant elements in the universe. In contrast, the solid Earth is dominated by silicon, oxygen, aluminum, iron, and calcium (Chapter 1). In other words, Earth is not a typical sample of the universe. Nor, as it turns out, is the cell a typical sample of the solid Earth. Fig. 2.12 shows the relative abundance by mass of chemical elements present in human cells after all the water has been removed. Note that just four elements—carbon (C), oxygen (O), hydrogen (H), and nitrogen (N)—constitute 94% of the total dry mass, and that the most abundant element is carbon. The elemental composition of human cells is typical of all cells. Human life, and all life as we know it, is based on carbon. Carbon molecules play such an important role in living organisms that carbon-containing molecules have a special name—they are called organic molecules. Their central role in life implies that there must be something very special about carbon, and there is. Carbon has the ability to combine with many other elements to form a wide variety of molecules, each specialized for the functions it carries out in the cell.
2.4.1 Carbon atoms form four covalent bonds.
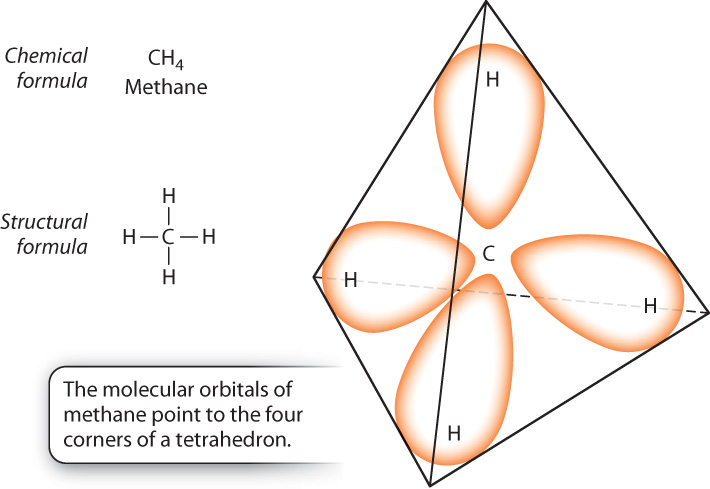
One of the special properties of carbon is that, in forming molecular orbitals, a carbon atom behaves as if it had four unpaired electrons. This behavior occurs because one of the electrons in the outermost sphere moves into the empty dumbbell-shaped orbital. In this process, the single large spherical orbital and three dumbbell-shaped orbitals change shape, becoming four equivalent hybrid orbitals each with one electron.
Fig. 2.13 shows the molecular orbitals that result when one atom of carbon combines with four atoms of hydrogen to form the gas methane (CH4). Each of the four valence electrons of carbon shares a new molecular orbital with the electron of one of the hydrogen atoms. These bonds can rotate freely about their axis. Furthermore, because of the shape of the orbitals, the carbon atom lies at the center of a three-dimensional structure called a tetrahedron, and the four molecular orbitals point toward the four corners of this structure. The ability of carbon to form four covalent bonds, the spatial orientation of these bonds in the form of a tetrahedron, and the ability of each bond to rotate freely all contribute importantly to the structural diversity of carbon-based molecules.
2.4.2 Carbon-based molecules are structurally and functionally diverse.
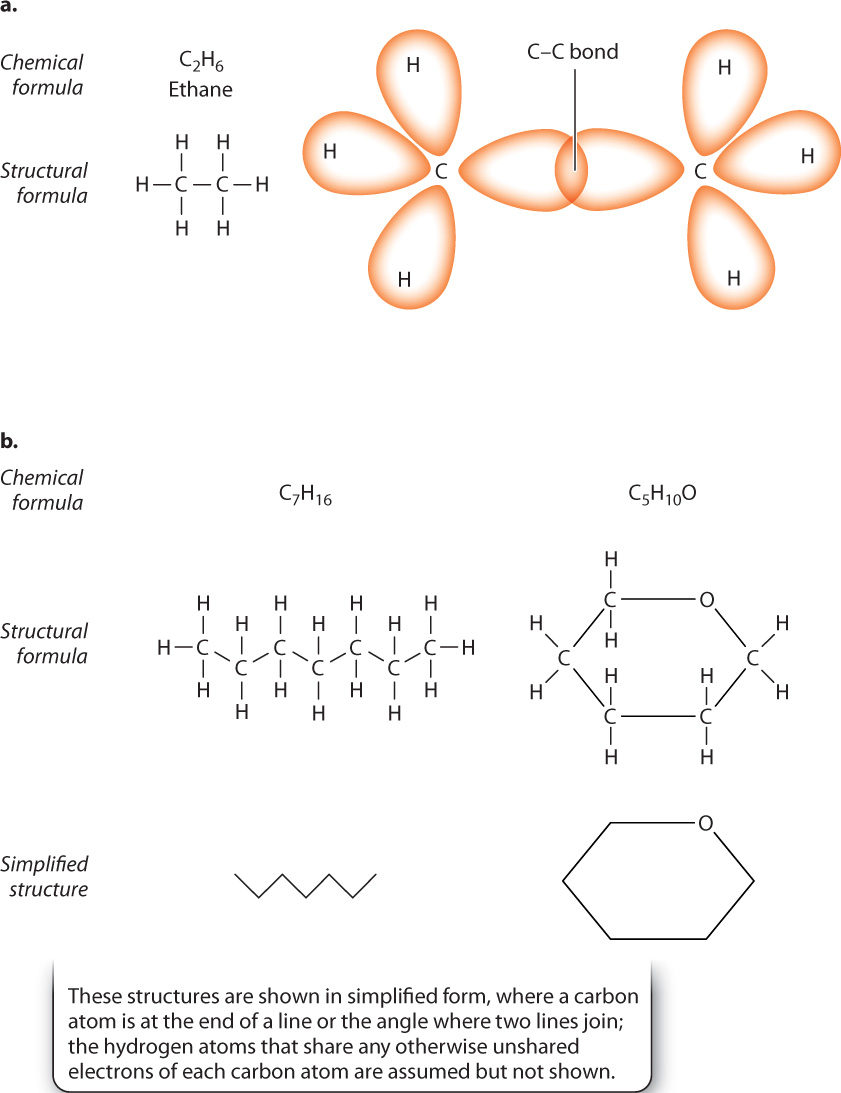
Carbon has other properties that contribute to its ability to form a diversity of molecules. For example, carbon atoms can link with each other by covalent bonds to form long chains. These chains can be branched, or two carbons at the ends of the chain or within the chain can link to form a ring structure.
Among the simplest chains is ethane, shown in Fig. 2.14a, in which two carbon atoms become connected by a covalent bond. In this case, the orbitals of unpaired electrons in two carbon atoms merge to create the covalent bond. Some more complex examples of carbon-containing molecules, and an abbreviated form, are shown in Fig. 2.14b.
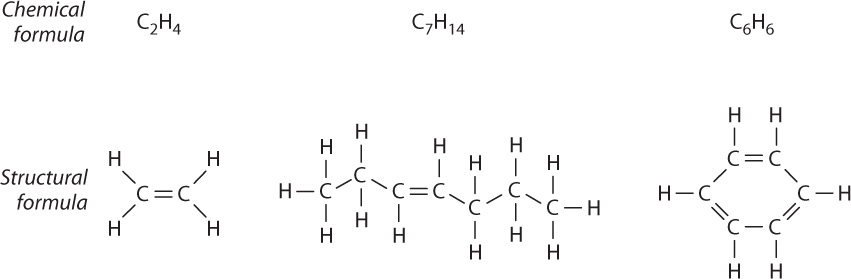
Two adjacent carbon atoms can also share two pairs of electrons, forming a double bond, as shown in Fig. 2.15. Note that each carbon atom has exactly four covalent bonds, but in this case two are shared between adjacent carbon atoms. The double bond is shorter than a single bond and is not free to rotate so that all of the covalent bonds in the carbon atoms connected by a double bond are in the same geometrical plane. As with single bonds, double bonds can be found in chains of atoms or cyclical structures. A double bond between atoms is represented by a double line connecting the two chemical symbols for the atoms.
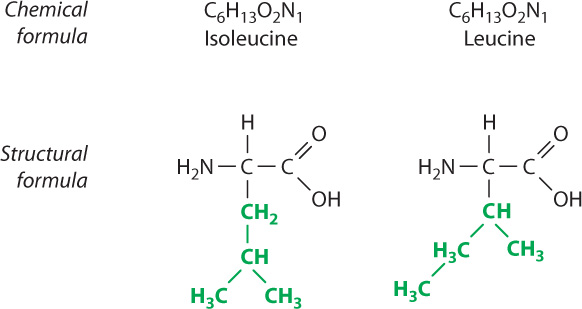
While the types of atoms making up a molecule help characterize the molecule, the spatial arrangement of atoms is also important. For example, 6 carbon atoms, 13 hydrogen atoms, 2 oxygen atoms, and 1 nitrogen atom can join covalently in many different arrangements to produce molecules with different structures. Two of these many arrangements are shown in Fig. 2.16. Note that some of the connections between atoms are identical (black) and some are different (green), even though the chemical formulas are the same (C6H13O2N1). Molecules that have the same chemical formula but different structures are known as isomers.
We have seen that carbon-containing molecules can adopt a wide range of arrangements, a versatility that helps us to understand how a limited number of elements can create an astonishing variety of molecules. We might ask whether carbon is uniquely versatile. Put another way, if we ever discover life on a distant planet, will it be carbon based, like us? Silicon, just below carbon in the periodic table (see Fig. 2.3), is the one other element that is both reasonably abundant and characterized by four atomic orbitals with one electron each. Some scientists have speculated that silicon might provide an alternative to carbon as a chemical basis for life. However, silicon readily binds oxygen. On Earth, nearly all of the silicon atoms found in molecules are covalently bound to oxygen. Studies of Mars and of meteorites show that silicon is tightly bound to oxygen throughout our solar system, and that is likely to be true everywhere we might explore. There are about 1000 different silicate minerals on Earth, but this diversity pales before the millions of known carbon-based molecules. If we ever discover life beyond Earth, very likely its chemistry will be based on carbon.