5.2 THE PLASMA MEMBRANE AND CELL WALL
All cells are enclosed by a plasma membrane, also called the cell membrane. The plasma membrane is a fundamental, defining feature of all cells. It is the boundary that defines the space of the cell, separating its internal contents from the surrounding environment. But the plasma membrane is not simply a passive boundary or wall. Instead, it serves an active and important function. The environment outside the cell is changing all the time. In contrast, the internal environment of a cell operates within a persistent and narrow window of conditions, such as pH or salt concentration. It is the plasma membrane that actively maintains intracellular conditions compatible with life.
In addition, the cells of many different groups of organisms have a cell wall external to the plasma membrane. The cell wall plays an important role in maintaining the shape and internal composition of these cells. In this section, we consider the functions performed by these two key structures.
5.2.1 The plasma membrane maintains homeostasis.
The active maintenance of a constant environment is known as homeostasis, and it is a critical attribute of cells and of life itself. Chemical reactions and protein folding, for example, are carried out efficiently only within a narrow range of conditions. How does the plasma membrane maintain homeostasis? The answer is that it acts as a selective barrier. This means that the plasma membrane lets some molecules in and out freely; it lets others in and out only under certain conditions; and it prevents still other molecules from passing through at all.
The membrane's ability to act as a selective barrier is the result of the combination of lipids and embedded proteins that make it up. The hydrophobic lipid bilayer prevents ions as well as charged or polar molecules from diffusing freely across the plasma membrane. Furthermore, many macromolecules such as proteins and polysaccharides are too large to cross the plasma membrane on their own. At the same time, gases, other lipids, and small polar molecules freely move across the lipid bilayer. Protein transporters in the membrane allow the export and import of molecules, including certain ions and nutrients, that cannot cross the cell membrane on their own.
The identity and abundance of these membrane-associated proteins vary among cell types, reflecting the specific functions of different cells. For example, cells in your gut contain membrane transporters that specialize in the uptake of glucose, while nerve cells have different types of ion channel that are involved in electrical signaling.
5.2.2 Passive transport involves diffusion.
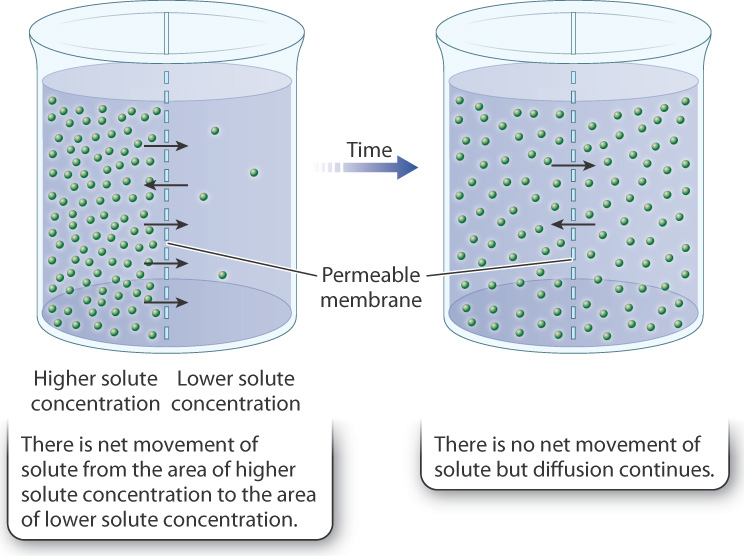
The simplest form of movement into and out of cells is passive transport. Passive transport works by diffusion, which is the random movement of molecules. When there is a concentration difference (that is, a concentration gradient) in the distribution of a molecule, with areas of higher and lower concentrations, diffusion results in net movement of the molecule from an area of higher concentration to an area of lower concentration (Fig. 5.9). However, diffusion occurs even in the absence of concentration differences, due to the random motion of molecules, but in this case there is no net movement of the substance.
Some molecules diffuse freely across the plasma membrane as a result of differences in concentrations between the inside and outside of a cell. Oxygen and carbon dioxide, for example, move into and out of the cell in this way. Certain hydrophobic molecules, such as triacylglycerols (Chapter 2), are also able to diffuse through the cell membrane, which is not surprising since the lipid bilayer is likewise hydrophobic.
Some molecules that cannot move across the lipid bilayer directly can move passively toward a region of lower concentration through protein channels or carriers. When a molecule moves by diffusion through a membrane protein and bypasses the lipid bilayer, the process is called facilitated diffusion. Diffusion and facilitated diffusion both result from the random motion of molecules, and net movement of the substance occurs when there are concentration differences (Fig. 5.10). In the case of facilitated diffusion, the molecule moves through a membrane protein channel or carrier, whereas in the case of simple diffusion, the molecule moves directly through the lipid bilayer.
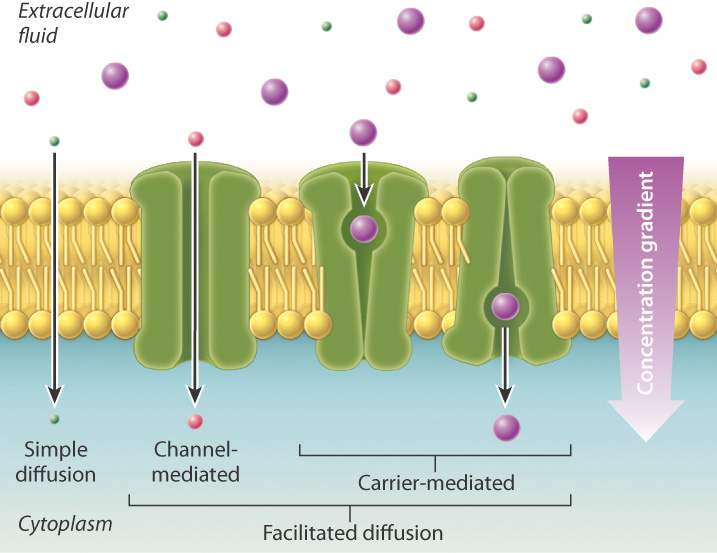
Although some membrane proteins provide a channel or opening between the inside and outside of the cell, most are carriers selective for specific molecules or are even "gated," opening and closing only in response to the binding of specific molecules or some other signal. These membrane proteins exist in two shapes or conformations, one that is open to one side of the cell, and another that is open to the other side of the cell. Binding of the transported molecule induces a conformational change in the membrane protein, allowing the molecule to be transported across the lipid bilayer, as shown on the right in Fig. 5.10.
Up to this point, we have focused our attention on the movement of molecules (solutes) in water (solvent). We can take a different perspective and focus instead on water movement. Water itself also moves into and out of cells by passive transport. Although the plasma membrane is hydrophobic, water molecules are small enough to move passively through the membrane to a limited extent by simple diffusion. In addition, many cells have specific protein channels, known as aquaporins, which allow water to flow through the plasma membrane more readily by facilitated diffusion.
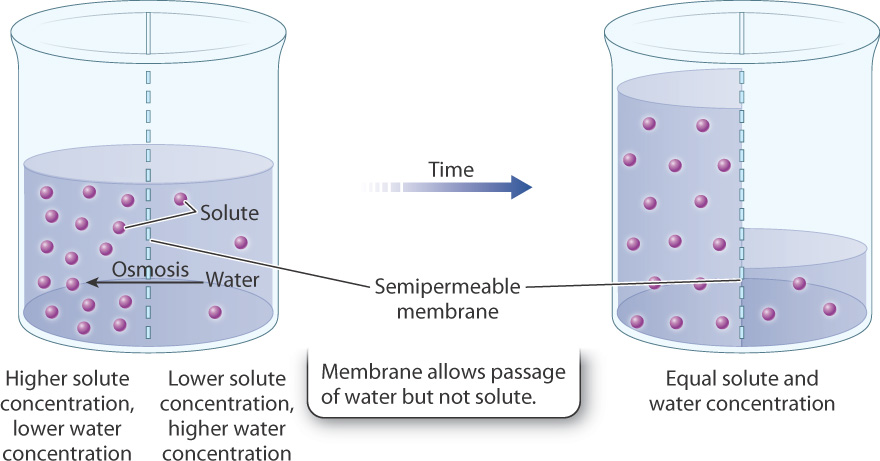
The diffusion of a solvent (such as water) across a selectively permeable membrane is known as osmosis. As in any form of diffusion, water moves from regions of higher water concentration to regions of lower water concentration (Fig. 5.11). Because water is a solvent within which nutrients such as glucose or ions such as sodium or potassium are dissolved, water concentration drops as solute concentration rises. Therefore, it is sometimes easier to think about water moving from regions of lower solute concentration to regions of higher solute concentration. Either way, the direction of water movement is the same.
Quick Check 2
A container is divided into two compartments by a membrane that is fully permeable to water and small ions. Water is added to one side of the membrane (side A), and a 5% solution of sodium chloride (NaCl) is added to the other (side B). In which direction will water molecules move? In which direction will sodium and chloride ions move? When the concentration is equal on both sides, will diffusion stop?
5.2.3 Primary active transport uses the energy of ATP.
Passive transport works to the cell’s advantage only if the concentration gradient is in the right direction, from higher on the outside to lower on the inside for nutrients that the cell needs to take in, and from higher on the inside and lower on the outside for wastes that the cell needs to export. However, many of the molecules that cells require are in low concentration in the environment. Although some of these molecules can be synthesized by the cell, others need to be taken up from the environment and concentrated inside the cell. In other words, cells have to move these substances from areas of lower concentration to areas of higher concentration. The "uphill" movement of substances against a concentration gradient, called active transport, requires energy. The transport of many kinds of molecules across membranes requires energy, either directly or indirectly. In fact, most of the energy used by a cell goes into keeping the inside of the cell different from the outside, a function carried out by proteins in the plasma membrane.
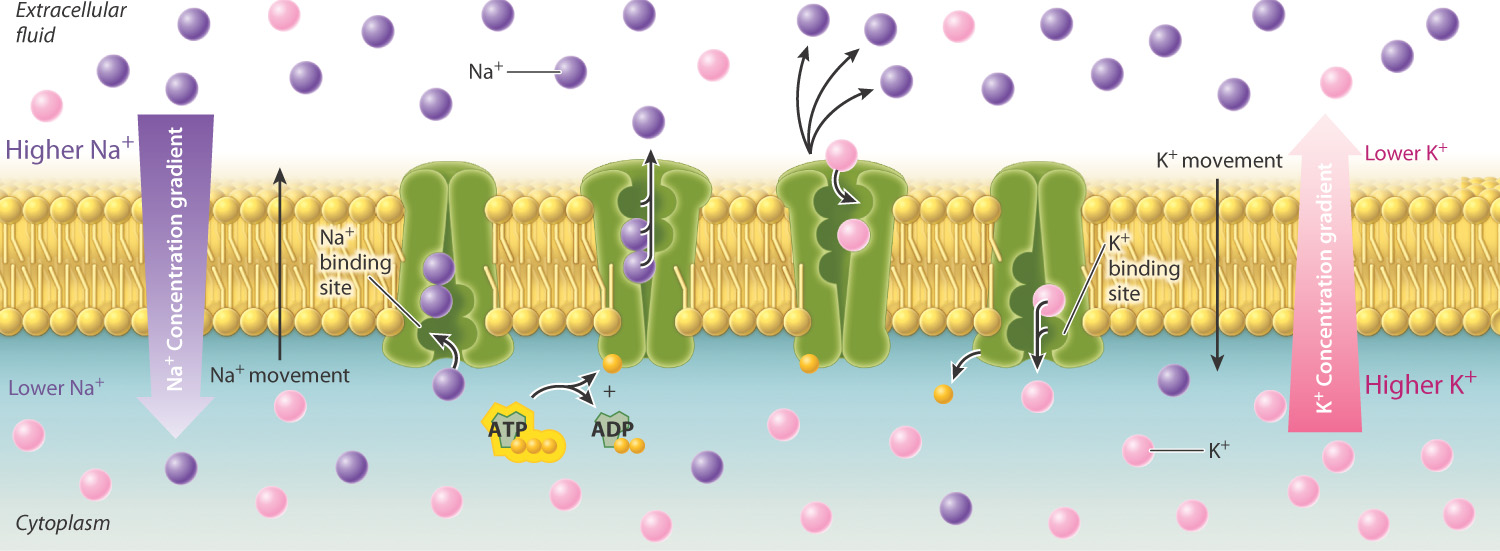
During active transport, cells move substances through transport proteins embedded in the cell membrane. Some of these proteins act as pumps, using energy directly to move a substance into or out of a cell. A good example is the sodium-potassium pump (Fig. 5.12). Within cells, sodium is kept at concentrations much lower than in the exterior environment; the opposite is true of potassium. Therefore, both sodium and potassium have to be moved against a concentration gradient. The sodium-potassium pump actively moves sodium out of the cell and potassium into the cell. This movement of ions takes energy, which comes from the chemical energy stored in ATP. Active transport that uses energy directly in this manner is called primary active transport. Note that the sodium and potassium ions move in opposite directions. Protein transporters that work in this way are sometimes referred to as antiporters. Other transporters move two molecules in the same direction. These transport proteins are referred to as symporters or cotransporters.
5.2.4 Secondary active transport is driven by an electrochemical gradient.
Active transport can also work in another way. Because small ions cannot cross the lipid bilayer, many cells use a transport protein to build up the concentration of a small ion on one side of the membrane. The resulting concentration gradient stores energy that can be harnessed to drive the movement of other substances across the membrane against their concentration gradient.
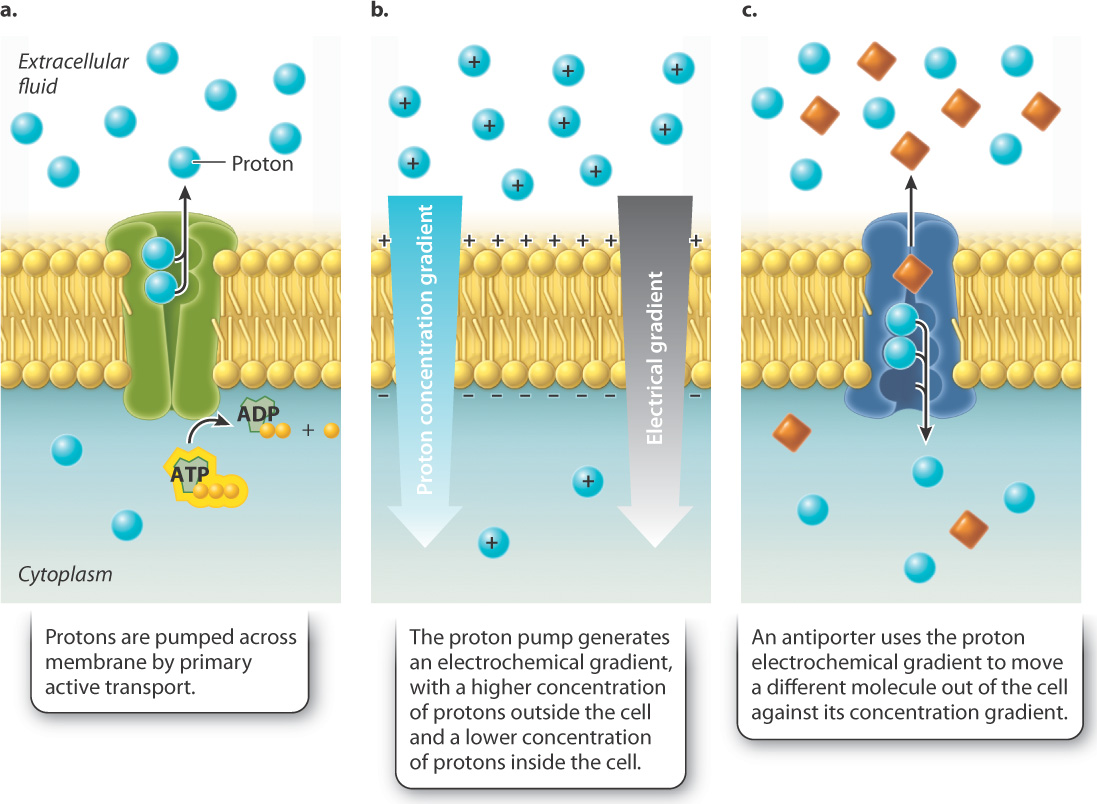
For example, some cells actively pump protons (H+) across the cell membrane using ATP (Fig. 5.13a). As a result, the concentration of protons is higher on one side of the membrane and lower on the other side. In other words, the pump generates a concentration gradient, also called a chemical gradient because the entity forming the gradient is a chemical (Fig. 5.13b). We have already seen that concentration differences favor the movement of protons back to the other side of the membrane. However, the lipid bilayer blocks the movement of protons to the other side and therefore stores energy, just like a dam or battery.
In addition to the chemical gradient, another force favors the movement of protons back across the membrane: a difference in charge. Because protons carry a positive charge, the side of the membrane with more protons is more positive than the other side. This difference in charge is called an electrical gradient. Protons (and other ions) move from areas of like charge to areas of unlike charge, driven by an electrical gradient. Together, the charge and chemical gradients are known as an electrochemical gradient (Fig. 5.13b).
If protons are then allowed to pass through the cell membrane by a transport protein, they will move down their electrochemical gradient toward the region of lower proton concentration. These transport proteins can use the movement of protons to drive the movement of other molecules against their concentration gradient (Fig. 5.13c). The movement of protons is always from regions of higher to lower concentration, while the movement of the coupled molecule is from regions of lower to higher concentration. Because the movement of the coupled molecule is driven by the movement of protons and not by ATP directly, this form of transport is called secondary active transport. Secondary active transport uses the energy of an electrochemical gradient to drive the movement of molecules; by contrast, primary active transport uses the energy of ATP directly.
The use of an electrochemical gradient as a temporary energy source is a common cellular strategy. For example, cells use the sodium electrochemical gradient generated by the sodium-potassium pump to transport glucose and amino acids into cells. In addition, cells use the proton electrochemical gradient to move other molecules and, as we discuss below and in Chapter 7, to synthesize ATP.
5.2.5 Many cells maintain size and composition using active transport.
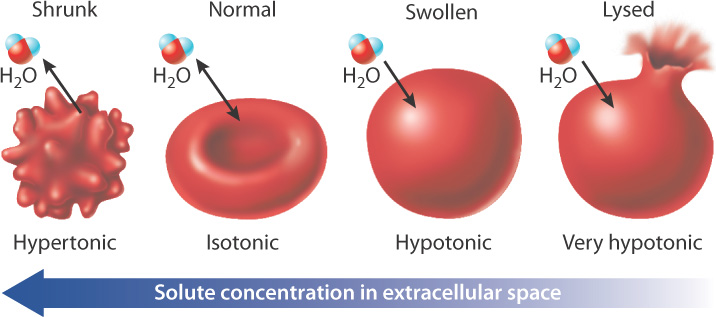
Many cells use active transport to maintain their size and composition. Consider human red blood cells placed in a variety of different solutions (Fig. 5.14). If a red blood cell is placed in a hypertonic solution (one with a higher solute concentration than that inside the cell), water leaves the cell by osmosis and the cell shrinks. By contrast, if a red blood cell is placed in a hypotonic solution (one with a lower solute concentration than that inside the cell), water moves into the cell by osmosis and the cell lyses, or bursts. Animal cells solve the problem of water movement in part by maintaining the intracellular fluid isotonic (that is, at the same solute concentration) as the extracellular fluid. Cells use the active transport of ions to maintain equal concentrations inside and out, and the sodium-potassium pump plays an important role in keeping the inside of the cell isotonic with the extracellular fluid.
Quick Check 3
In the absence of the sodium-potassium pump, the extracellular solution becomes hypotonic relative to the inside of the cell. Poisons such as the snake venom ouabain can interfere with the action of the sodium-potassium pump. What are the consequences for the cell?
Human red blood cells avoid shrinking or bursting by maintaining an intracellular environment isotonic with the extracellular environment, the blood. But what about a single-celled organism, like Paramecium, swimming in a freshwater lake? In this case, the extracellular environment is hypotonic compared to the concentration in the cell’s interior. As a result, Paracemium faces the risk of bursting caused by water moving in by osmosis. Paramecium and some other single-celled organisms contain contractile vacuoles that solve this problem. Contractile vacuoles are compartments that take up excess water from inside the cell and then, by contraction, expel it into the external environment. The mechanism by which water moves into the contractile vacuole differs depending on the organism. The contractile vacuole of some organisms takes in water through aquaporins, while other organisms have proton pumps that first move protons into the contractile vacuole, with water following by osmosis.
5.2.6 The cell wall provides another means of maintaining cell shape.
Most organisms do not contain contractile vacuoles. How do organisms such as plants, fungi, and bacteria maintain cell size and composition? A key feature of these organisms is the cell wall, a rigid structure that surrounds the plasma membrane (Fig. 5.15). The cell wall plays a critical role in the maintenance of cell shape and volume. When Hooke looked at cork through his microscope, what he saw was not living cells but the remains of cell walls.
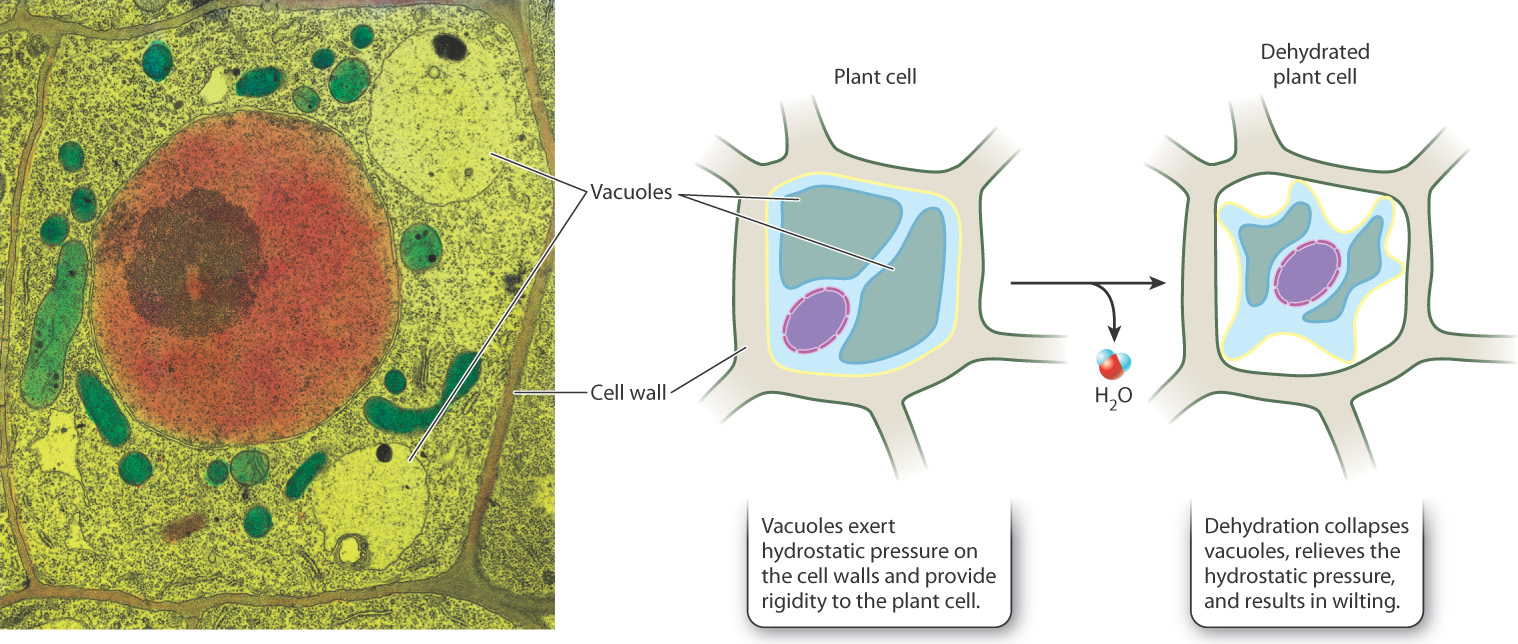
The cell wall provides structural support and protection to the cell. Because the cell wall is fairly rigid and resists expansion, it allows pressure to build up in a cell when it absorbs water. The force exerted by water pressing against an object results in hydrostatic pressure, or turgor pressure. The pressure exerted by water provides structural support for many organisms that is similar in function to the support provided by the skeletons or shells of animals. In addition, many plant cells have another structure, called the vacuole (distinct from the contractile vacuole), that also absorbs water and contributes to turgor pressure (Fig. 5.15). It is therefore easy to understand why plants wilt when dehydrated—the loss of water from the vacuoles reduces turgor pressure and the tissue can no longer maintain rigidity.
The cell wall is made up of many different components, including carbohydrates and proteins. The specific components differ depending on the organism. The plant cell wall is composed of polysaccharides, one of the best known of which is cellulose, a polymer of the sugar glucose. Cellulose is the most abundant biological material in nature. Many types of algae have cell walls made up of cellulose, as in plants. Fungi have cell walls made of chitin, another polymer based on sugars. In bacteria, the cell wall is made up primarily of peptidoglycan, a mixture of amino acids and sugars. Animal cells do not have walls.