6.2 ENERGY
The energy of a system has a formal definition: It is the system’s capacity to do work. For a cell, this work involves processes we discussed in earlier chapters, such as synthesizing DNA, RNA, and proteins, pumping substances into and out of the cell, and moving vesicles between various compartments of a cell. The sun is a familiar source of energy, and we recognize energy in movement. On a cellular level, energy is held in chemical bonds, such as those of ATP, glucose, and lipids. How do these molecules contain energy in their chemical bonds? In this section, we discuss different sources of energy.
6.2.1 Kinetic and potential energy are two forms of energy.
Light, electricity, wind, and fossil fuels are all different sources of energy, and they can be classified as one of two types: kinetic energy or potential energy (Fig. 6.3). Kinetic energy is the energy of motion, and it is perhaps the most familiar form of energy. Moving objects possess kinetic energy because they perform work, resulting in the movement of themselves and the surrounding matter. For example, a ball bouncing down a set of stairs possesses kinetic energy. Kinetic energy is associated with any kind of movement, such as a person running or a muscle contracting. Similarly, light is associated with the movement of photons, electricity with the movement of electrons, and thermal energy (perceived as heat) with the movement of molecules, so these, too, are forms of kinetic energy.
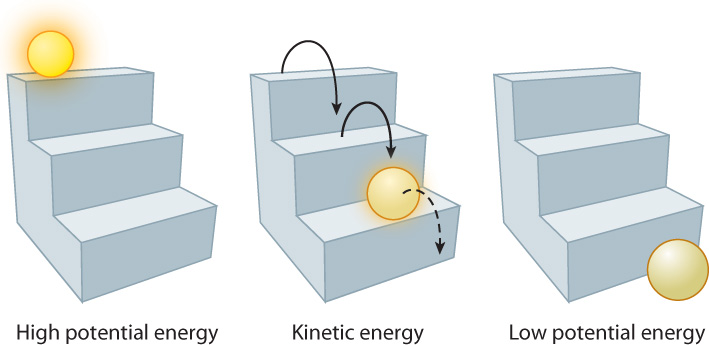
Energy is not always associated with motion. An immobile object can still possess a form of energy called potential energy, or stored energy. Potential energy depends on the structure of the object or its position relative to its surroundings, and it is released by a change in the object’s structure or position. For example, the potential energy of a ball is higher at the top of a flight of stairs than at the bottom (Fig. 6.3). If it were not blocked by the floor, it would move from the position of higher potential energy (the top of the stairs) to the position of lower potential energy (the bottom of the stairs) because of the force of gravity. Similarly, an electrochemical gradient of molecules across a cell membrane is a form of potential energy. Given the chance, the molecules move down their concentration and electrical gradients (Chapter 5).
Energy can be converted from one form to another. The ball at the top of the stairs has a certain amount of potential energy because of its position. As it rolls down the stairs, this potential energy is converted to kinetic energy associated with movement of the ball and the surrounding air. When the ball reaches the bottom of the stairs, the remaining energy is stored as potential energy. Conversely, it takes an input of energy to move the ball back to the top of the stairs, and this input of energy is stored as potential energy.
6.2.2 Chemical energy is a form of potential energy.
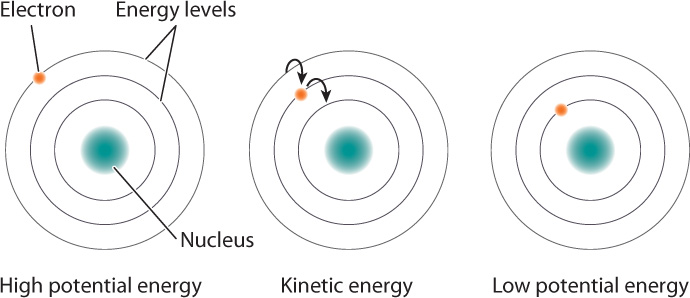
We obtain the energy we need from the food we eat, which contains chemical energy, a form of potential energy. How do food molecules contain potential energy? Recall from Chapter 2 that atoms consist of a nucleus with positively charged protons and electrically neutral neutrons, and negatively charged electrons occupying orbitals at various distances from the nucleus. Like the ball on the stairs, electrons have potential energy based on their position. The farther away an electron is from the nucleus, the more potential energy it has (Fig. 6.4). Given the chance, an electron will move closer to the nucleus because of the attraction between the positively charged nucleus and the negatively charged electron. When electrons move closer to the nucleus, some of this potential energy is converted to other types of energy, such as heat or light energy. Conversely, it takes an input of energy to move electrons farther from the nucleus, and this energy is stored as potential energy.
Recall, too, that a covalent bond results from the sharing of the electrons between two atoms. When these shared electrons are far from both nuclei, they have a large amount of potential energy; when close, they have less potential energy. In this way, molecules carry potential energy in their chemical bonds. Organic molecules contain many covalent bonds, including carbon–carbon (C–C) bonds and carbon–hydrogen (C–H) bonds. In these bonds, the electrons are far from the two atoms, so they store a large amount of chemical potential energy. Organic molecules such as carbohydrates, lipids, and proteins are rich sources of chemical energy and therefore are sometimes called fuel molecules.
6.2.3 ATP is the cell's energy currency.
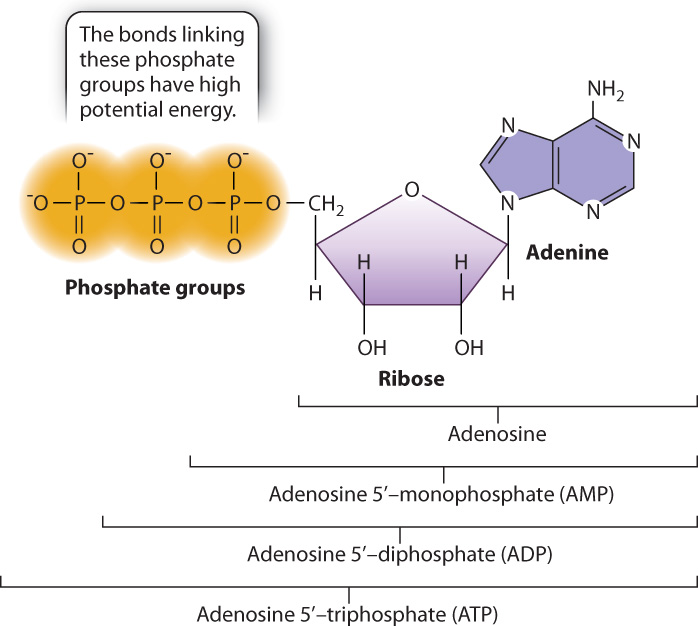
The chemical energy carried in carbohydrates, lipids, and proteins is harnessed by cells to do work. But cells do not use this energy all at once. Instead, through a series of chemical reactions described in Chapter 7, they package this energy into a chemical form that is readily accessible to the cell. One form of chemical energy is adenosine triphosphate, or ATP, shown in Fig. 6.5. The chemical energy in the bonds of ATP is used in turn to drive many cellular processes, such as muscle contraction, cell movement, and membrane pumps. In this way, ATP serves as a “go-between,” acting as an intermediary between fuel molecules that store a large amount of potential energy in their bonds and the activities of the cell that require an input of energy.
ATP is composed of adenosine, which is made up of the base adenine and the five-carbon sugar ribose, attached to triphosphate, or three phosphate groups (Fig. 6.5). Its chemical relatives are ADP (adenosine diphosphate) and AMP (adenosine monophosphate), with two phosphate groups and one phosphate group, respectively. The use of ATP as an energy source in nearly all cells reflects its use early in the evolution of life on Earth.
The chemical energy of ATP is held in the bonds connecting the phosphate groups. At physiological pH, these phosphate groups are negatively charged and have a tendency to repel each other. The chemical bonds connecting the phosphate groups therefore store potential energy that is released when the bonds are broken. In turn, the released energy can power the work of the cell.