6.4 CHEMICAL REACTIONS
Living organisms build and break down molecules, pump ions across membranes, move, and in general function largely through chemical reactions. As we have seen, organisms break down food molecules, storing energy in the bonds of ATP, which then powers chemical reactions that sustain life. Chemical reactions are therefore central to life processes. We describe here several features of a chemical reaction.
6.4.1 A chemical reaction occurs when molecules interact.
As we saw in Chapter 2, a chemical reaction is the process by which molecules, called reactants, are transformed into other molecules, called products. During a chemical reaction, atoms keep their identity, but the bonds linking the atoms change. For example, carbon dioxide (CO2) and water (H2O) can react to produce carbonic acid (CH2O3), as shown here and in Fig. 6.8:
CO2 + H2O → H2CO3
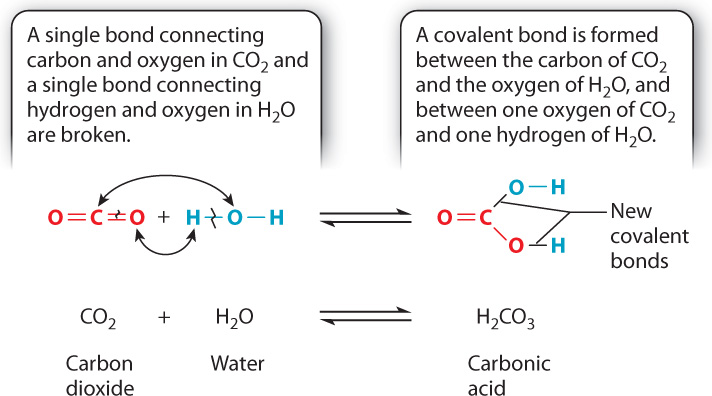
The preceding reaction occurs commonly. For example, in an aqueous (watery) environment, carbonic acid exists as bicarbonate ions (HCO3−) and protons (H+). This reaction occurs in the oceans, and explains why the oceans are becoming more acidic with increasing carbon dioxide levels in the atmosphere. In addition, when cells break down glucose in organisms, they produce carbon dioxide. In animals, this carbon dioxide diffuses out of cells and into the blood. Rather than remaining dissolved in solution, most of the carbon dioxide is converted into carbonic acid according to the preceding reaction.
Most chemical reactions in cells are reversible; the products can react to form the reactants. For example, carbon dioxide and water react to form carbonic acid, and carbonic acid can also dissociate to produce carbon dioxide and water. This reverse reaction also occurs in the blood, allowing carbon dioxide to be removed from the lungs or gills (Chapter 39). The reversibility of the reaction is indicated by a double arrow:
CO2 + H2O ⇋ H2CO3
The way the reaction is written defines the forward and reverse reactions: A forward reaction proceeds from left to right and the reactants are placed on the left side of the arrow; a reverse reaction proceeds from right to left and the reactants are placed on the right side of the arrow.
The direction of a reversible reaction can be influenced by the concentrations of reactants and products. For example, increasing the concentration of the reactants or decreasing the concentration of the products favors the forward reaction. This effect explains how many reactions in metabolic pathways proceed: The products of many reactions are quickly consumed by the next reaction, helping to drive the first reaction forward.
6.4.2 Chemical reactions are subject to the laws of thermodynamics.
Chemical reactions, like all processes in the cell, are subject to the laws of thermodynamics. We have seen that cells use chemical reactions to perform much of the work of the cell. The amount of energy available to do work is called Gibbs free energy (G). In a chemical reaction, we can compare the free energy of the reactants and products to determine whether there is energy available to do work. The difference between two values is denoted by the Greek letter delta (Δ). In this case, ΔG is the free energy of the products minus the free energy of the reactants (Fig. 6.9). If the products of a reaction have more free energy than the reactants, then ΔG is positive and a net input of energy is required to drive the reaction forward (Fig. 6.9a). By contrast, if the products of a reaction have less free energy than the reactants, ΔG is negative and energy is released and available to do work (Fig. 6.9b).
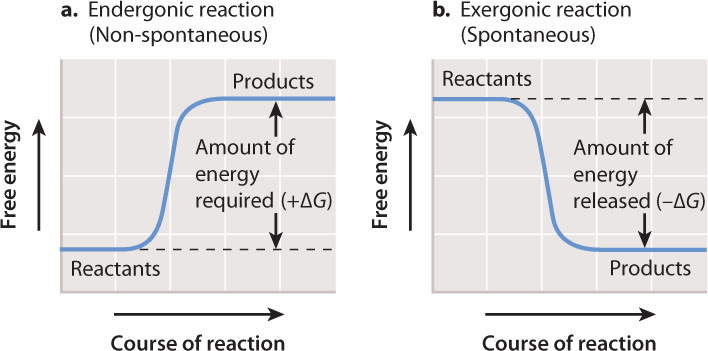
Reactions with a negative ΔG that release energy and proceed spontaneously are called exergonic, and reactions with a positive ΔG that require an input of energy and are not spontaneous are called endergonic. Note that the term “spontaneous” does not imply instantaneous or even rapid. “Spontaneous” in this context means that a reaction releases energy; “non-spontaneous” means that a reaction requires a sustained input of energy.
Recall that the total amount of energy is equal to the energy available to do work plus the energy that is not available to do work because of the increase in disorder. We can write this relationship as an equation, in which the total amount of energy is enthalpy (H), the energy available to do work is Gibbs free energy (G), and the degree of disorder is entropy (S) multiplied by the absolute temperature (T) (measured in degrees Kelvin), since temperature influences the movement of molecules (and hence the degree of disorder). Therefore,
Total amount of energy (H) = energy available to do work (G) + energy lost to entropy (TS)
We are interested in the energy available for a cell to do work, or G. Therefore, we express G in terms of the other two parameters, H and TS, as follows:
G = H − TS
In a chemical reaction, we can compare the total energy and entropy of the reactants with the total energy and entropy of the products to see if there is energy available to do work. As a result, we get
ΔG = ΔH − T ΔS
This equation is a useful way to see if a chemical reaction takes place, what direction it proceeds in, and whether net energy is required or released. Let’s take a step back and make sure it makes sense intuitively. The value of ΔG depends on both the change in enthalpy and the change in disorder. Catabolic reactions are those in which the products have less chemical energy in their bonds than in those of the reactants and in which the products are more disordered than the reactants. In other words, such reactions have a negative value of ΔH and a positive value of ΔS, and therefore a negative value of ΔG and proceed spontaneously (Fig. 6.10). The reverse is also true: Increasing chemical potential energy and decreasing disorder, as in the synthesis of proteins from individual amino acids and other anabolic reactions, results in a positive value of ΔG and requires a net input of energy.
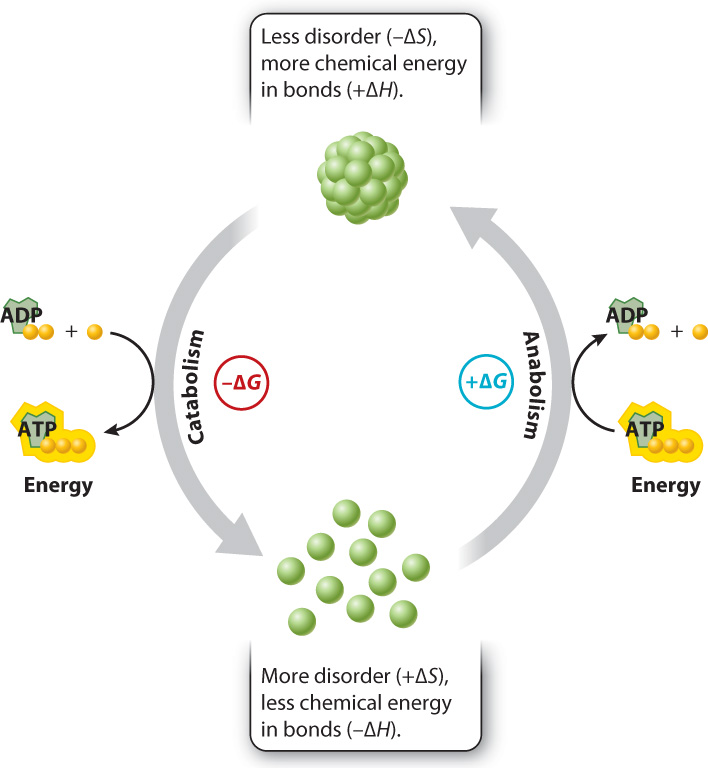
There are also cases in which the change in enthalpy and the change in entropy are both positive or both negative. In these cases, the absolute value of these parameters determines whether ΔG is positive or negative and therefore whether a reaction is spontaneous or not.
Quick Check 2
How does increasing the temperature affect the change in free energy (ΔG) of a chemical reaction?
6.4.3 The hydrolysis of ATP releases energy.
Let’s apply these concepts to a specific chemical reaction. Earlier, we introduced ATP, the molecule that drives many cellular processes using the chemical potential energy in its chemical bonds. ATP reacts with water to lose a phosphate group (HPO42−), forming ADP and inorganic phosphate (Pi) (Fig. 6.11):
ATP + H2O → ADP + Pi
This is an example of a hydrolysis reaction, a chemical reaction in which a water molecule is split into a proton (H+) and a hydroxyl group (OH–). Hydrolysis reactions often break down polymers into their subunits, and in the process one product gains a proton and the other gains a hydroxyl group.
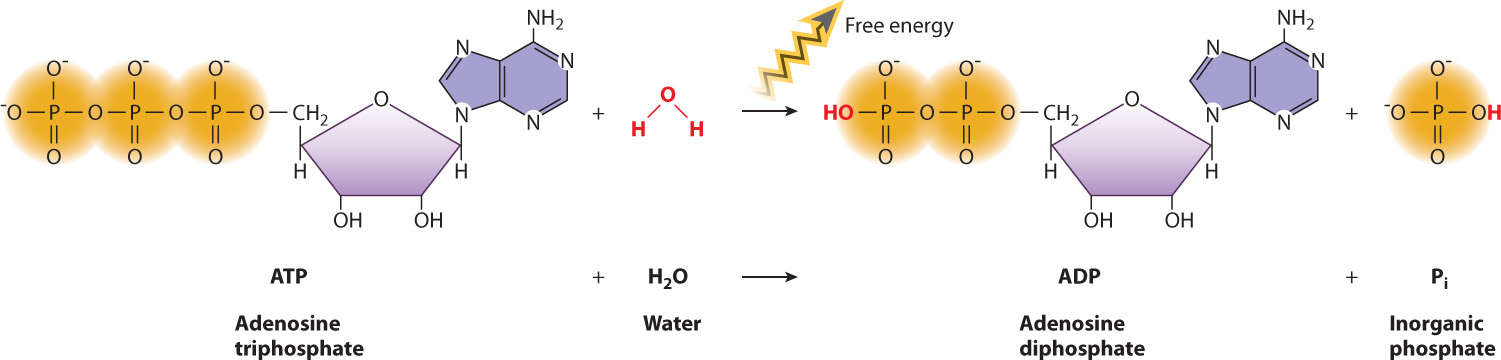
The reaction of ATP with water is an exergonic reaction that releases energy. This negative free energy difference between the products and reactants can be explained by referring to the formula we derived in the last section. Recall that the phosphate groups of ATP are negatively charged at physiological pH and repel each other. ATP has three phosphate groups, and ADP has two. Therefore, ADP contains less chemical potential energy in its bonds than does ATP, resulting in a negative value of ΔH. In addition, a single molecule of ATP is broken down into two molecules, ADP and Pi. Therefore, the reaction is also associated with an increase in entropy, or a positive value of ΔS. Since ΔG = ΔH - T ΔS, ΔG is negative and the reaction is a spontaneous one that releases free energy. The release of free energy during the hydrolysis of ATP allows ATP to drive chemical reactions and other processes that require a net input of energy, as we will see next.
6.4.4 Non-spontaneous reactions are often coupled to spontaneous reactions.
If the conversion of reactant A into product B is spontaneous, the reverse reaction leading to the formation of B from A is not. The ΔG’s for the forward and reverse reactions have the same absolute value but opposite signs. You might expect that the direction of the reaction would always be from A to B. However, in a living organism, not all chemical reactions are spontaneous. Anabolic reactions are a good example; they require an input of energy to drive them in the right direction. This raises the question: What drives non-spontaneous reactions? The coupling of a non-spontaneous reaction to a spontaneous one can drive a non-spontaneous reaction, as long as the net ΔG of the two reactions is negative.
Let’s consider the following two reactions:
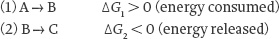
Only reaction 2 is spontaneous since ΔG2 < 0, but if the sum of ΔG1 and ΔG2 is negative, then the overall reaction A →→ C is spontaneous and the transformation of A into B occurs despite a positive ΔG. This type of energetic coupling, in which a spontaneous reaction drives a non-spontaneous one, provides the thermodynamic driving force of a non-spontaneous biochemical reaction.
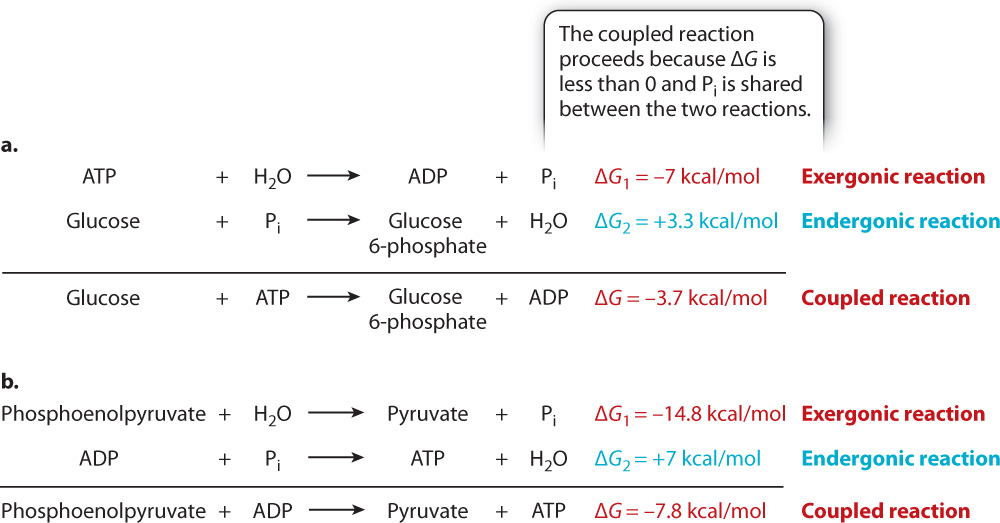
In a cell, the most common reaction involving energetic coupling is the hydrolysis of ATP. This reaction can drive an endergonic reaction that requires an input of energy if the two reactions are coupled together (Fig. 6.12a). Coupled reactions involving ATP share a phosphate group, which is transferred between ATP and other molecules in the reaction.
Following ATP hydrolysis, the cell needs to replenish its ATP so that it can carry out additional chemical reactions. The synthesis of ATP from ADP and Pi is an endergonic reaction with a positive ΔG, requiring an input of energy. In some cases, exergonic reactions can drive the synthesis of ATP by energetic coupling (Fig. 6.12b). The sum of the ΔG’s of the two reactions is negative, allowing the overall reaction to proceed.
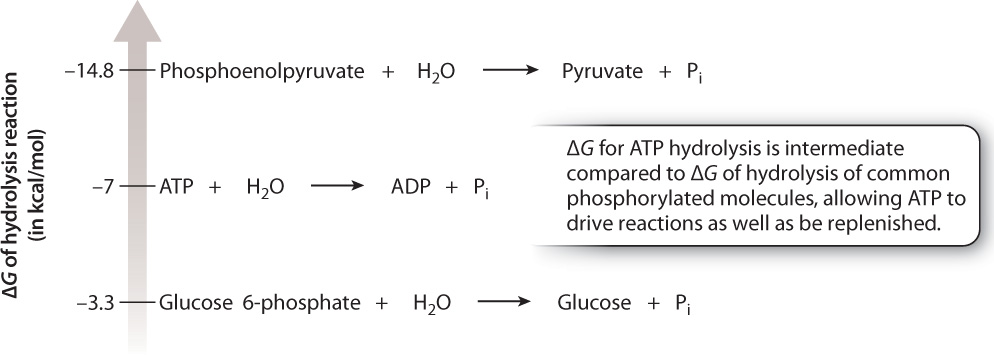
Phosphorylated molecules, like ATP, can be hydrolyzed, releasing free energy. Hydrolysis reactions can be ranked by their free energy differences. Fig. 6.13 shows that ATP hydrolysis has an intermediate free energy difference compared to the free energy difference for hydrolysis of other common phosphorylated molecules. Those reactions that have a ΔG more negative than that of ATP hydrolysis give a phosphate group to ADP, and those reactions that have a ΔG less negative than that of ATP hydrolysis receive a phosphate group from ATP by energetic coupling. Therefore, ADP is an energy acceptor and ATP an energy provider. The ATP–ADP system is at the core of energetic coupling between catabolic and anabolic reactions. In this way, ATP continually turns over in a cell.