6.5 ENZYMES AND THE RATE OF CHEMICAL REACTIONS
Up to this point, we have focused on the spontaneity and direction of chemical reactions. Now we address their rate. As mentioned earlier, a spontaneous reaction is not necessarily a fast one. For example, the breakdown of glucose into carbon dioxide and water is spontaneous with a negative ΔG, but the rate of the reaction is close to zero and the breakdown of glucose is imperceptible. However, glucose is readily broken down inside cells all the time. How is this possible? The answer is that chemical reactions in a cell are accelerated by chemical catalysts.
The rate of a chemical reaction is defined as the amount of product formed (or reactant consumed) per unit of time. Catalysts are substances that increase the rate of chemical reactions without themselves being consumed. In biological systems, the catalysts are usually proteins called enzymes, although, as we saw in Chapter 2, some RNA molecules have catalytic activity as well. Enzymes can increase the rate of chemical reactions dramatically. Moreover, because they are highly specific, acting only on certain reactants and catalyzing only some reactions, enzymes play a critical role in determining which chemical reactions take place from all the possible reactions that could occur in a cell. In this section, we discuss how enzymes increase the rate of chemical reactions and how that ability gives them a central role in metabolism.
6.5.1 Enzymes reduce the activation energy of a chemical reaction.
Earlier, we saw that exergonic reactions release free energy and endergonic reactions require free energy. Nevertheless, all chemical reactions require an input of energy to proceed, even exergonic reactions that release energy. For this type of reaction, the energy released is more than the initial input of energy, so there is a net release of energy.
Why is an input of energy needed for all chemical reactions? As a chemical reaction proceeds, existing chemical bonds break and new ones form. For an extremely brief period of time, a compound is formed in which the old bonds are breaking and the new ones are forming. This intermediate stage between reactants and products is called the transition state. It is highly unstable and therefore has a large amount of free energy.
In all chemical reactions, reactants adopt at least one transition state before their conversion into products. The graph in Fig. 6.14 represents the free energy levels of the reactant, transition state, and product. This reaction is spontaneous since the free energy of the reactant is higher than the free energy of the product and ΔG is negative. However, the highest free energy value corresponds to the transition state.
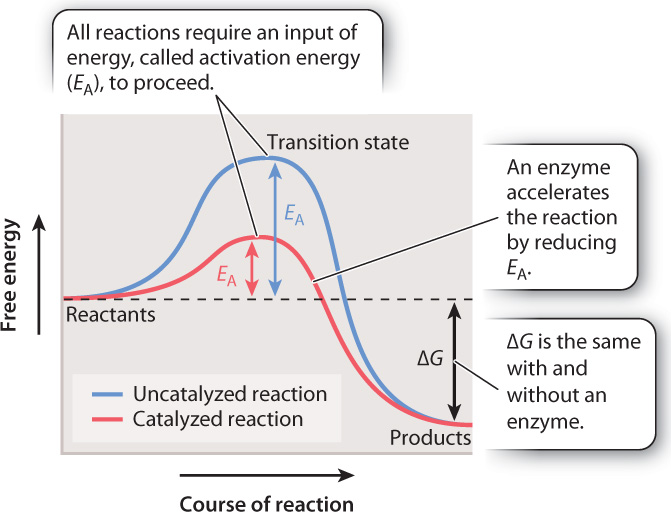
To reach the transition state, the reactant must absorb energy from its surroundings (the uphill portion of the curve in Fig. 6.14). As a result, all chemical reactions, even spontaneous ones that release energy, require an input of energy that we can think of as an “energy barrier.” The energy input necessary to reach the transition state is called the activation energy (EA). Once the transition state is reached, the reaction proceeds, products are formed, and energy is released into the surroundings (the downhill portion of the curve in Fig. 6.14). There is an inverse correlation between the rate of a reaction and the height of the energy barrier: the lower the energy barrier, the faster the reaction; the higher the barrier, the slower the reaction.
In chemical reactions that take place in the lab, heat is a common source of energy used to overcome the energy barrier. In living organisms, reactions are accelerated by the action of enzymes. However, enzymes do not act by supplying heat. Instead, they reduce the activation energy by stabilizing the transition state and decreasing its free energy (the red line in Fig. 6.14). As the activation energy decreases, the speed of the reaction increases.
Although an enzyme accelerates a reaction by reducing the activation energy, the difference in free energy between reactants and products (ΔG) does not change. In other words, an enzyme changes the path of the reaction between reactants and products, but not the starting or end point (Fig. 6.14). Consider the breakdown of glucose into carbon dioxide and water. The ΔG of the reaction is the same whether it proceeds by combustion or by the action of multiple enzymes in a metabolic pathway in a cell.
Quick Check 3
Which of the following do enzymes change?
ΔG, reaction rate; types of products generated; activation energy; the laws of thermodynamics.
6.5.2 Enzymes form a complex with reactants and products.
An important characteristic of enzymes is that, as catalysts, they participate in a chemical reaction but are not themselves consumed in the process. In other words, they emerge unchanged from a chemical reaction, ready to catalyze the same reaction again. How do enzymes increase the reaction rate without being consumed? The answer is that enzymes form a complex with the reactants and products.
In an uncatalyzed reaction, a reactant, often called a substrate (S), is converted to a product (P):
S ⇋ P
In the presence of an enzyme (E), the substrate first forms a complex with the enzyme (enzyme–substrate, or ES). While still part of the complex, the substrate is converted to product (enzyme–product, or EP). Finally, the complex dissociates, releasing the enzyme and product. Therefore, a reaction catalyzed by an enzyme can be described as
S + E ⇋ ES ⇋ EP ⇋ E + P
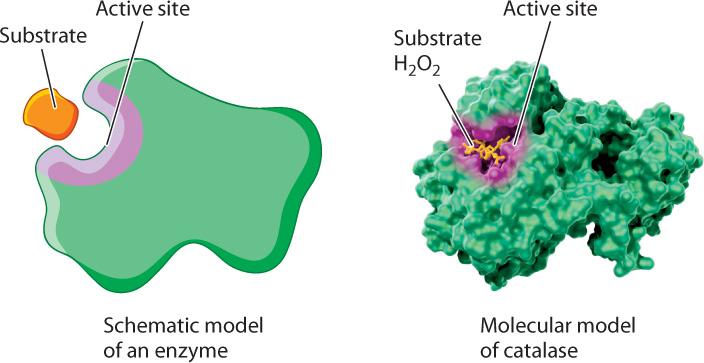
The formation of this complex is critical for accelerating the rate of a chemical reaction. Recall from Chapter 4 that proteins adopt three-dimensional shapes and that the shape of a protein is linked to its function. Enzymes are folded into three-dimensional shapes that bring particular amino acids into proximity to form an active site. The active site of the enzyme is the portion of the enzyme that binds substrate and converts it to product (Fig. 6.15). In the active site, the enzyme and substrate form both transient covalent bonds and/or weak noncovalent interactions. Together, these interactions stabilize the transition state and decrease the activation energy.
Enzymes also reduce the energy of activation by positioning two substrates to react. The formation of the enzyme–substrate complex promotes the reaction between two substrates by aligning their reactive chemical groups and limiting their motion relative to each other.
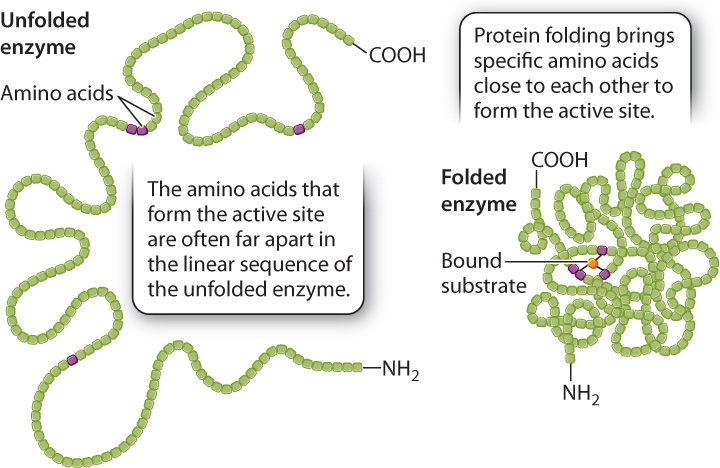
The size of the active site is extremely small compared to the size of the enzyme. If only a small fraction of the enzyme is necessary for the catalysis of a reaction, why are enzymes so large? Of the many amino acids that form the active site, only a few actively contribute to catalysis. Each of these amino acids has to occupy a very specific spatial position to align with the correct reactive group on the substrate. If the few essential amino acids were part of a short peptide, the alignment of chemical groups between the peptide and the substrate would be difficult or even impossible because the length of the bonds and the bond angles in the peptide would constrain its three-dimensional structure. In fact, in many cases the catalytic amino acids are spaced far apart in the primary structure of the enzyme, but brought close together in the formation of the active site by protein folding (Fig. 6.16). In other words, the large size of many enzymes is required at least in part to bring the catalytic amino acids into very specific positions in the active site of the folded enzyme.
The formation of a complex between enzymes and substrates can be demonstrated experimentally, as illustrated in Fig. 6.17 and Fig. 6.18, using two different techniques to answer the same question.
FIG. 6.17Do enzymes form complexes with substrates?
BACKGROUND The idea that enzymes form complexes with substrates to catalyze a chemical reaction was first proposed in 1888 by the Swedish chemist Svante Arrhenius. One of the earliest experimental demonstrations in support of this idea was made by American chemist Kurt Stern. He studied an enzyme called catalase, which is very abundant in animal and plant tissues. Catalase converts hydrogen peroxide (H2O2), which is toxic to cells, to water (H2O) and oxygen (O2):

Catalase is very efficient, able to convert hydrogen peroxide to water and oxygen very rapidly.
HYPOTHESIS Stern hypothesized that catalase forms a complex with hydrogen peroxide.
METHOD Stern used spectral analysis to test his hypothesis. He shined a white light on the reaction as it took place. Different molecules absorb different wavelengths of light. The maximum absorption of a wavelength by each different molecule is called the absorption peak. By analyzing the patterns of absorption peaks as the reaction proceeded, Stern could determine whether or not an intermediate complex was formed.
RESULTS A schematic representation of the absorption peaks is shown here. Note the appearance of a new absorption peak in panel II, consistent with the formation of an enzyme–substrate complex.
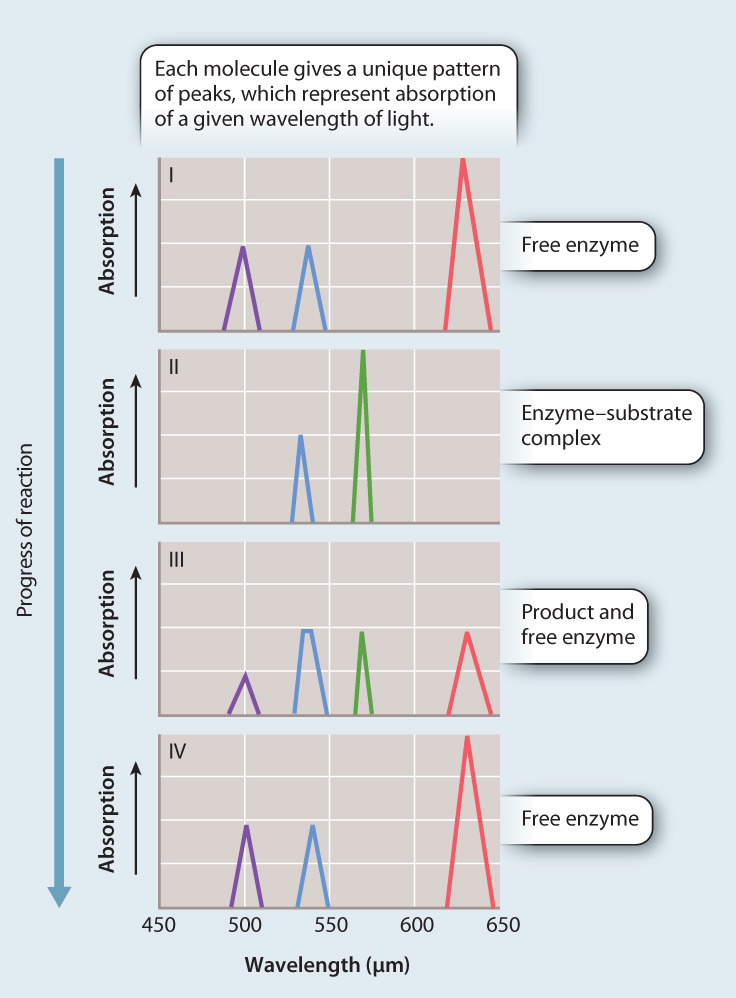
CONCLUSION The simplest interpretation of the data is that catalase forms a complex with hydrogen peroxide during its conversion to water and oxygen, providing support for the initial hypothesis.
FOLLOW-UP WORK As Stern wrote, “It remains to be seen to which extent the findings of this study apply to enzyme action in general.” In other words, the next steps were to see if other enzymes also work by forming a complex with their substrates.
SOURCE Stern, K. G. 1936. “On the Mechanism of Enzyme Action: A Study of the Decomposition of Monoethyl Hydrogen Peroxide by Catalase and of an Intermediate Enzyme–Substrate Compound.” J. Biol. Chem. 114:473–494.
FIG. 6.18Do enzymes form complexes with substrates?
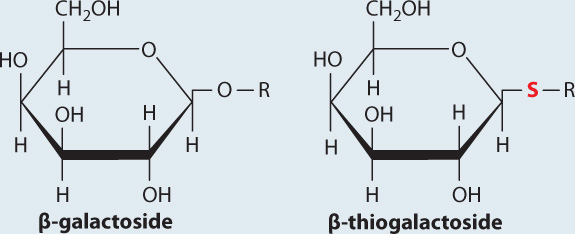
BACKGROUND The enzyme β-galactosidase catalyzes the cleavage of the glycosidic bond that links galactose to glucose in the disaccharide lactose. Lactose belongs to a family of molecules called β-galactosides since it contains a galactose unit attached to the rest of the molecule by a glycosidic bond. In a related compound called β-thiogalactoside, the oxygen atom in the glycosidic bond is replaced by sulfur. The enzyme β-galactosidase binds β-thiogalactoside but cannot cleave or release it.
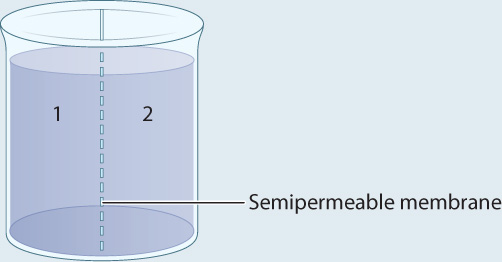
METHOD A container is separated into two compartments by a semipermeable membrane. The membrane is permeable to β-galactoside and β-thiogalactoside, but not permeable to the enzyme.
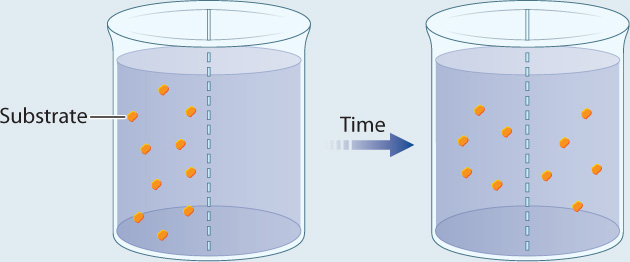
EXPERIMENT 1 Radioactively labeled β-thiogalactoside (S) is added to compartment 1 and the movement of S is followed by measuring the level of radioactivity in the two compartments.
RESULT Over time, the level of radioactivity is the same in the two compartments.
EXPERIMENT 2 Radioactively labeled β-thiogalactoside (S) is added to compartment 1, enzyme (E) is added to compartment 2, and the movement of S is followed by measuring the level of radioactivity in the two compartments.
RESULT Over time, the level of radioactivity is greater in compartment 2 than in compartment 1.
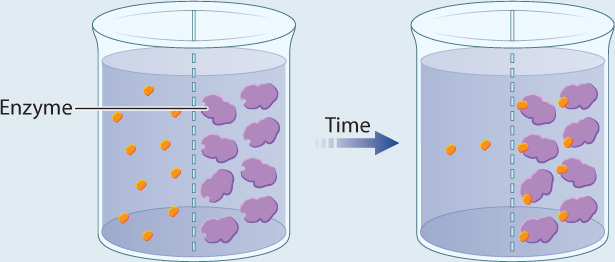
CONCLUSION These results can be explained if the substrate diffuses from compartment 1 to compartment 2, forms a complex with the enzyme, and is not released. In other words, E and S form a complex.
SOURCE Adapted from Doherty, D. G., and F. Vaslow. 1952. “Thermodynamic Study of an Enzyme–Substrate Complex of Chymotrypsin.” J. Am. Chem. Soc. 74:931–936.
6.5.3 Enzymes are highly specific.
Enzymes are remarkably specific both for the substrate and the reaction that is catalyzed. In general, enzymes recognize either a unique substrate or a class of substrates that share common chemical structures. In addition, enzymes catalyze only one reaction or a very limited number of reactions.
For example, the enzyme succinate dehydrogenase acts only on succinate, whereas the enzyme (β-(beta-) galactosidase is specific for a class of molecules. The enzyme (β-galactosidase catalyzes the cleavage of the glycosidic bond that links galactose to glucose in the disaccharide lactose, as well as any glycosidic bond that links galactose to one of several types of molecule. In this case, the enzyme does not recognize the whole substrate, but a particular structural motif within it, and very small differences in the structure of this motif affect the activity of the enzyme. For example, (β-galactosidase cleaves (β-galactoside but does not cleave α-(alpha-) galactoside, which differs from (β-galactoside only in the orientation of the glycosidic bond. Hence, the enzyme is able to discriminate between two identical bonds with different orientations within a molecule. The specificity of enzymes can be attributed to the structure of their active sites. The enzyme active site interacts only with substrates having a precise three-dimensional structure.
6.5.4 Enzyme activity can be influenced by inhibitors and activators.
The activity of enzymes can be influenced by inhibitors and activators. Inhibitors decrease the activity of enzymes, whereas activators increase the activity of enzymes. Enzyme inhibitors are quite common. They are synthesized naturally by many plants and animals as a defense against predators. Similarly, pesticides and herbicides often target enzymes to inactivate them. Many drugs used in medicine are enzyme inhibitors, including drugs used to treat infections as well as cancer and other diseases. Given the importance of chemical reactions and the role of enzymes in metabolism, it is not surprising that enzyme inhibitors have such widespread applications.
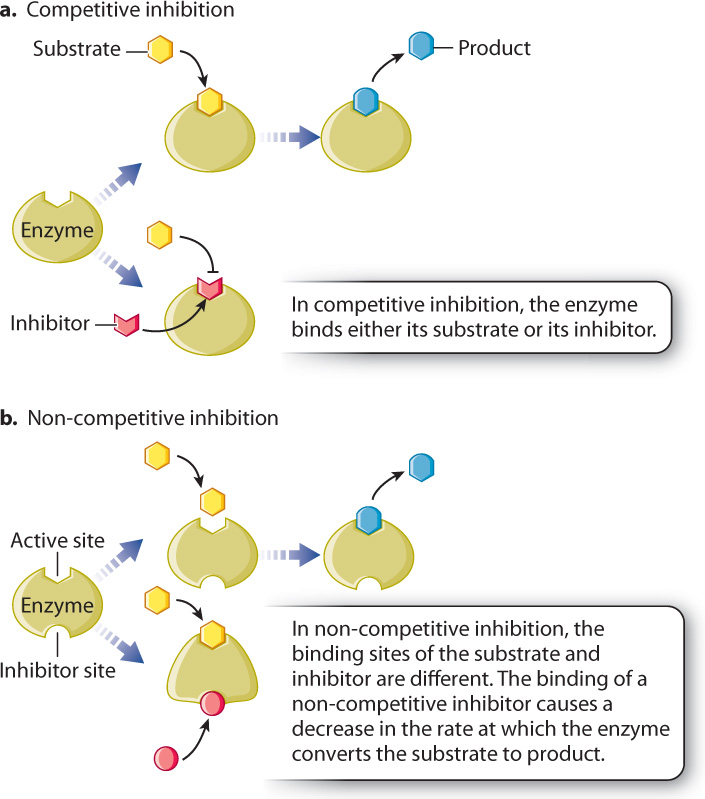
Inhibitors bind either to the active site of the enzyme or elsewhere, but in both cases they hinder the enzyme’s ability to accelerate the conversion of reactants to products. Inhibitors fall into two classes. Irreversible inhibitors usually form covalent bonds with enzymes and irreversibly inactivate them. Reversible inhibitors form weak bonds with enzymes and easily dissociate from them.
Reversible inhibitors can act competitively or non-competitively with enzymes (Fig. 6.19). Competitive inhibitors bind to the active site of the enzyme and prevent the binding of the substrate (Fig. 6.19a). In other words, they compete with substrate for the active site of the enzyme. Competitive inhibitors are often structurally similar to the substrate. By binding to the active site of the enzyme, competitive inhibitors reduce the affinity of the enzyme for the substrate. Therefore, competitive inhibitors can be overcome by increasing the concentration of substrate.
Non-competitive inhibitors usually have a structure very different from that of the substrate and bind to the enzyme at a site different from the active site (Fig. 6.19b). When the inhibitor is bound, the enzyme is still able to bind the substrate and there is no competition between the binding of the substrate and the inhibitor. Therefore, the affinity of the enzyme for its substrate is unchanged by a non-competitive inhibitor. However, a non-competitive inhibitor slows down the reaction by altering the shape of the enzyme and reducing its activity.
6.5.5 Allosteric enzymes in the cell are regulated by activators and inhibitors.
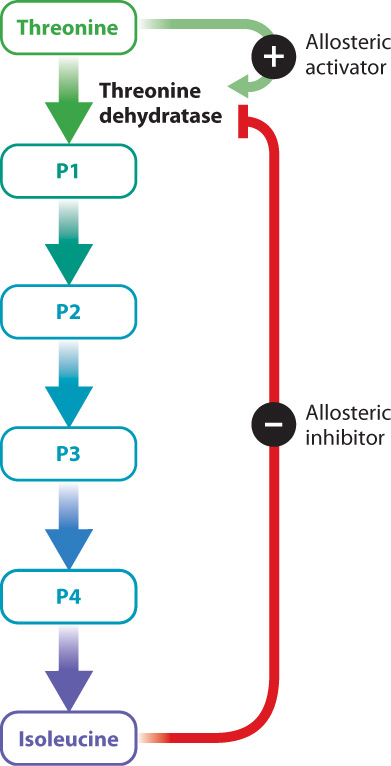
Enzyme activators and inhibitors are sometimes important in the normal operation of a cell—for example, to regulate a metabolic pathway. Consider the synthesis of isoleucine from threonine, a pathway found in some bacteria. This conversion requires five reactions, each catalyzed by a different enzyme (Fig. 6.20). Once the bacterium has enough isoleucine for its needs, it would be a waste of energy to continue synthesizing the amino acid. To shut down the pathway once it is no longer needed, the cell relies on an enzyme inhibitor. The inhibitor is isoleucine, the final product of the five reactions. Isoleucine binds to the first enzyme in the pathway, threonine dehydratase, at a site distinct from the active site. The binding of isoleucine changes the shape of the enzyme and in this way inhibits its function. This is an example of negative feedback, in which the final product inhibits the first step of the reaction.
Threonine dehydratase is an example of an allosteric enzyme. Allosteric enzymes bind to activators and inhibitors at sites that are different from the active site, resulting in a change in the shape and activity of the enzyme. Threonine dehydratase is allosterically inhibited by isoleucine, as we have seen. In addition, it is allosterically activated by the first substrate in the series of reactions, threonine (Fig. 6.20). Threonine binds to the enzyme and changes its conformation in a way that increases its activity. At a low concentration of threonine, the rate of the reaction is very slow. As the concentration of threonine increases, the activity of the enzyme increases. At a particular threshold, a small increase in threonine concentration results in a large increase in reaction rate. Finally, when there is excess substrate, the reaction rate slows down. In effect, the allosteric enzyme adjusts the rate of a reaction depending on substrate concentration.
In the next two chapters, we examine more closely two key metabolic processes, cellular respiration and photosynthesis. Both of these processes require many chemical reactions acting in a coordinated fashion. Allosteric enzymes catalyze key reactions in metabolism and are usually found at or near the start of a metabolic pathway or at the crossroads between two metabolic pathways. Allosteric enzymes are one way that the cell coordinates the activity of multiple metabolic pathways.
Case 1 The First Cell: Life's Origins
6.5.6 What naturally occurring elements might have spurred the first reactions that led to life?
Many enzymes are not just made up of protein, but also contain metal ions. These ions are one type of cofactor, a substance that associates with an enzyme and plays a key role in its function. Metallic cofactors, especially iron, magnesium, manganese, cobalt, copper, zinc, and molybdenum, bind to diverse proteins, including those used in DNA synthesis, nitrogen metabolism, and the transport of electrons for cellular respiration and photosynthesis, processes that are discussed in the next two chapters. With this in mind, scientists have asked whether metal ions might, by themselves, catalyze chemical reactions thought to have played a role in the origin of life. They do. For example, magnesium and zinc ions added to solutions can accelerate the linking of nucleotides to form RNA and DNA molecules.
Enzymes that contain iron and sulfur clustered together are particularly important in the transport of electrons within cells. Iron–sulfur minerals, especially pyrite (or fool’s gold, FeS2), form commonly in mid-ocean hydrothermal vent systems and other environments where oxygen is absent. It has been proposed that reactions now carried out in cells by iron–sulfur proteins reflect chemical reactions that took place spontaneously on the early Earth. The idea that your cells preserve an evolutionary memory of ancient hydrothermal environments may seem like science fiction, but it finds support in laboratory experiments. For example, the reaction of H2S and FeS to form pyrite has been shown to catalyze a number of plausibly pre-biotic chemical reactions, including the formation of pyruvate (a key intermediate in energy metabolism discussed in Chapter 7). Thus, the metals in enzymes help connect the chemistry of life to the chemistry of Earth.