7.5 THE ELECTRON TRANSPORT CHAIN AND OXIDATIVE PHOSPHORYLATION
The complete oxidation of glucose during glycolysis and the citric acid cycle results in the production of two kinds of electron carriers: NADH and FADH2. We are now going to see how the energy stored in these electron carriers is used to synthesize ATP.
The energy in these electron carriers is released in a series of redox reactions that occur as electrons pass through a chain of protein complexes in the inner mitochondrial membrane to the final electron acceptor, oxygen, which is reduced to water. The energy in these electrons is not converted directly into the chemical energy of ATP, however. Instead, the passage of electrons is coupled to the transfer of protons (H+) across the inner mitochondrial membrane, creating a concentration and charge gradient (Chapter 5). This electrochemical gradient provides a source of potential energy that is then used to drive the synthesis of ATP.
We next explore the properties of the electron transport chain, the proton gradient, and the synthesis of ATP.
7.5.1 The electron transport chain transfers electrons and pumps protons.
Electrons are not directly transported from NADH and FADH2 to oxygen. Instead, they are transported along a series of four large protein complexes that form the electron transport chain (complexes I to IV). These are shown in Fig. 7.9. These membrane proteins are embedded in the mitochondrial inner membrane (see Fig. 7.5). The inner mitochondrial membrane contains one of the highest concentrations of proteins found in eukaryotic membranes.
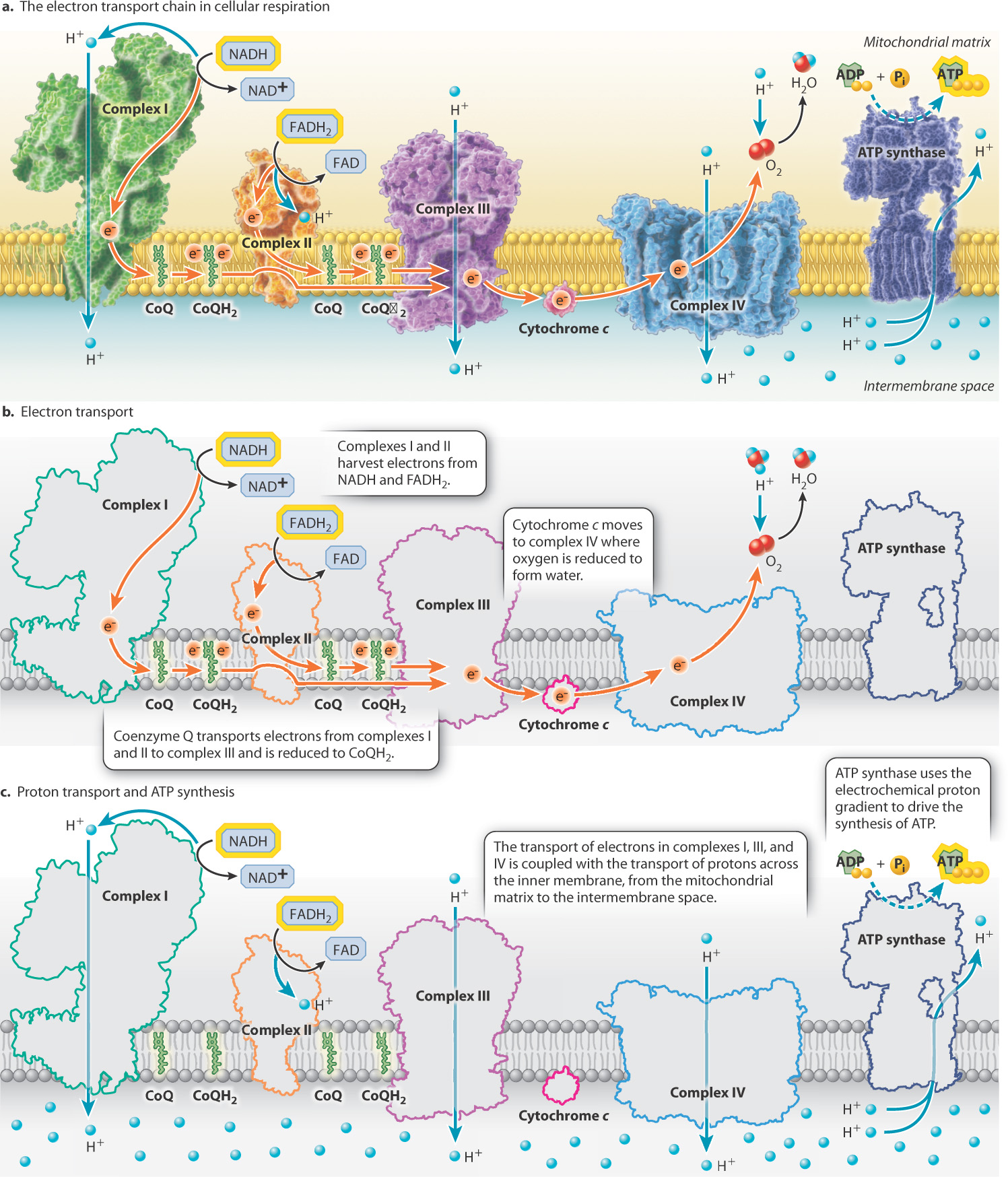
Electrons enter the electron transport chain via either complex I or II. Electrons donated by NADH enter through complex I, and electrons donated by FADH2 enter through complex II (complex II is the same enzyme that catalyzes step 6 in the citric acid cycle). These electrons are transported through either complex I or II to complex III and then through complex IV.
Within each protein complex of the electron transport chain, electrons are passed from electron donors to electron acceptors. Each donor and acceptor is a redox couple, consisting of an oxidized and a reduced form of a molecule. The electron transport chain contains many of these redox couples. As electrons are passed from donors to acceptors, the energy of the electrons is reduced. Each electron acceptor therefore binds electrons more strongly than the previous one in the chain. Oxygen is the final electron acceptor, and it has the highest affinity for electrons. When oxygen accepts an electron, it is reduced to water.
Electrons also must be transported between the four complexes (Fig. 7.9). Coenzyme Q (CoQ), also called ubiquinone, accepts electrons from both complexes I and II. When it accepts an electron, CoQ is reduced to CoQH2, which diffuses in the inner membrane, docks, and transfers electrons to complex III. Complex III in turn transfers electrons to cytochrome c. When it accepts an electron, cytochrome c is reduced, diffuses in the membrane, and interacts with complex IV.
These electron transfer steps are each associated with the release of energy as electrons are passed from the high-energy electron carriers NADH and FADH2 to the final low-energy (high-affinity) electron acceptor, oxygen. Some of this energy is used to reduce the next carrier in the chain, but in complexes I, III, and IV some of it is used to pump protons (H+) across the inner mitochondrial membrane, from the mitochondrial matrix to the intermembrane space (Fig. 7.9). Thus, the transfer of electrons through complexes I, III, and IV is coupled with the pumping of protons. The result is an accumulation of protons in the intermembrane space.
Quick Check 4
Animals breathe in air that contains more oxygen than the air they breathe out. Where is oxygen consumed?
7.5.2 The proton gradient is a source of potential energy.
Like all membranes, the inner mitochondrial membrane is selectively permeable: Protons cannot passively diffuse across this membrane, and the movement of other molecules is controlled by transporters and channels (Chapter 5). We have just seen that the movement of electrons through membrane-embedded protein complexes is coupled with the pumping of protons from the mitochondrial matrix into the intermembrane space. The consequence is a proton gradient, a difference in proton concentration across the inner membrane.
The proton gradient has two components: a chemical gradient due to the difference in concentration and an electrical gradient due to the difference in charge between the two sides of the membrane. To reflect the dual contribution of the concentration gradient and the electrical gradient, the proton gradient is also called an electrochemical gradient.
The proton gradient is a source of potential energy, as discussed in Chapters 5 and 6. It stores energy much in the same way that a battery or a dam does. Through the actions of the electron transport chain, protons have a high concentration in the intermembrane space and a low concentration in the mitochondrial matrix. As a result, there is a tendency for protons to diffuse back to the mitochondrial matrix, driven by a difference in concentration and charge on the two sides of the membrane. This movement, however, is blocked by the membrane, so the gradient holds potential energy. That energy can be harnessed if a pathway is opened through the membrane because, as we will see shortly, the resulting movement of the protons through the membrane can be used to perform work.
In sum, the oxidation of the electron carriers NADH and FADH2 formed during glycolysis, acetyl-CoA synthesis, and the citric acid cycle leads to the generation of a proton electrochemical gradient, which is a source of potential energy. This source of potential energy is used to synthesize ATP.
7.5.3 ATP synthase converts the energy of the proton gradient into the energy of ATP.
In 1961, Peter Mitchell proposed a hypothesis to explain how the energy stored in the proton electrochemical gradient is used to synthesize ATP. In 1978, he was awarded the Nobel Prize in Chemistry for work that fundamentally changed the way we understand how energy is harnessed by a cell.
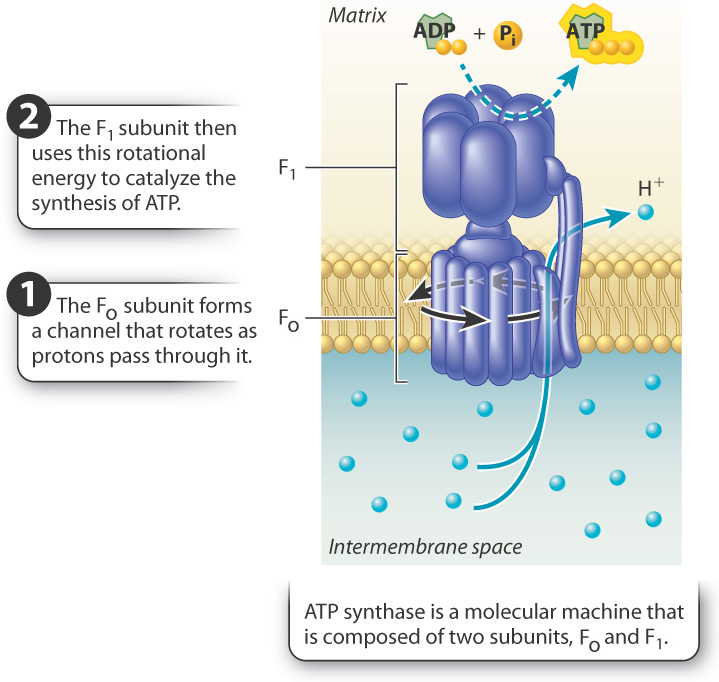
According to Mitchell’s hypothesis, the gradient of protons provides a source of potential energy that is converted into chemical energy stored in ATP. First, for the potential energy of the proton gradient to be released, there must be an opening in the membrane for the protons to flow through. Mitchell suggested that protons in the intermembrane space diffuse down their electrical and concentration gradients through a transmembrane protein channel into the mitochondrial matrix. Second, the movement of protons through the enzyme must be coupled with the synthesis of ATP. This coupling is made possible by ATP synthase, a remarkable enzyme composed of two distinct subunits called Fo and F1 (Fig. 7.10). Fo forms the channel in the inner mitochondrial membrane through which protons flow; F1 is the catalytic unit that synthesizes ATP. Proton flow through the channel (Fo) makes it possible for the enzyme (F1) to synthesize ATP.
Proton flow through the Fo channel causes it to rotate, converting the energy of the proton gradient into mechanical rotational energy, a form of kinetic energy. The rotation of the Fo subunit leads to rotation of the F1 subunit in the mitochondrial matrix (Fig. 7.10). The rotation of the F1 subunit in turn causes conformational changes that allow it to catalyze the synthesis of ATP from ADP and Pi. In this way, mechanical rotational energy is converted into the chemical energy of ATP.
Direct experimental evidence for Mitchell’s hypothesis did not come for over a decade. One of the key experiments that provided support for Mitchell’s hypothesis is illustrated in Fig. 7.11.
FIG. 7.11Can a proton gradient drive the synthesis of ATP?
BACKGROUND Peter Mitchell’s hypothesis that a proton gradient can drive the synthesis of ATP was proposed before experimental evidence supported it and was therefore met with skepticism. In the 1970s, biochemist Efraim Racker and his collaborator Walther Stoeckenius tested the hypothesis.
EXPERIMENT Racker and Stoeckenius built an artificial system consisting of a membrane, a bacterial proton pump activated by light, and ATP synthase.
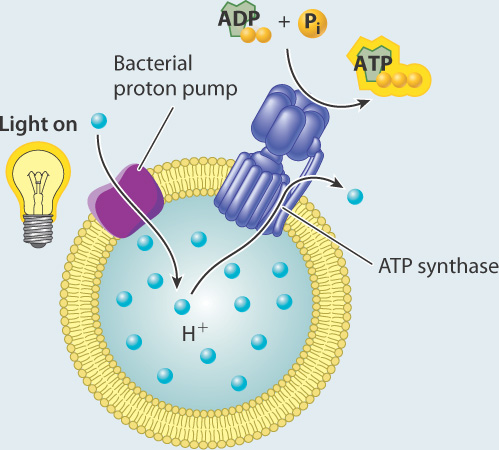
They measured the concentration of protons in the external medium and the amount of ATP produced in the presence and absence of light.
RESULTS In the presence of light, the concentration of protons increased inside the vesicles, suggesting that protons were taken up by the vesicles.
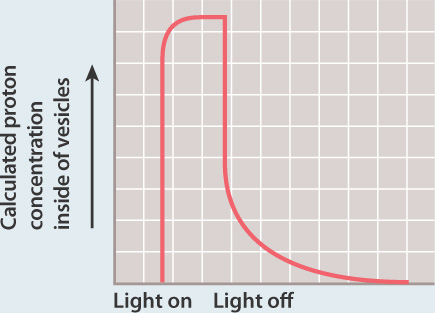
In the dark, the concentration of protons returned to the starting level. ATP was generated in the light, but not in the dark.

INTERPRETATION In the presence of light, the proton pump was activated and protons were pumped to one side of the membrane, leading to the formation of a proton gradient. The proton gradient, in turn, powered synthesis of ATP via ATP synthase.
CONCLUSION A membrane, proton gradient, and ATP synthase are sufficient to synthesize ATP. This result provided experimental evidence for Mitchell’s hypothesis.
SOURCES Mitchell, P. 1961. “Coupling of Phosphorylation to Electron and Hydrogen Transfer by a Chemiosmotic type of Mechanism.” Nature 191:144–148; Racker, E., AND W. Stoeckenius. 1974. “Reconstitution of Purple Membrane Vesicles Catalyzing Light-Driven Proton Uptake and Adenosine Triphosphate Formation.” J. Biol. Chem. 249?:?662–663.
Quick Check 5
Uncoupling proteins are proteins spanning the inner mitochondrial membrane that allow protons to pass through the membrane and bypass the channel of ATP synthase. Describe the consequences to the proton gradient and ATP production.
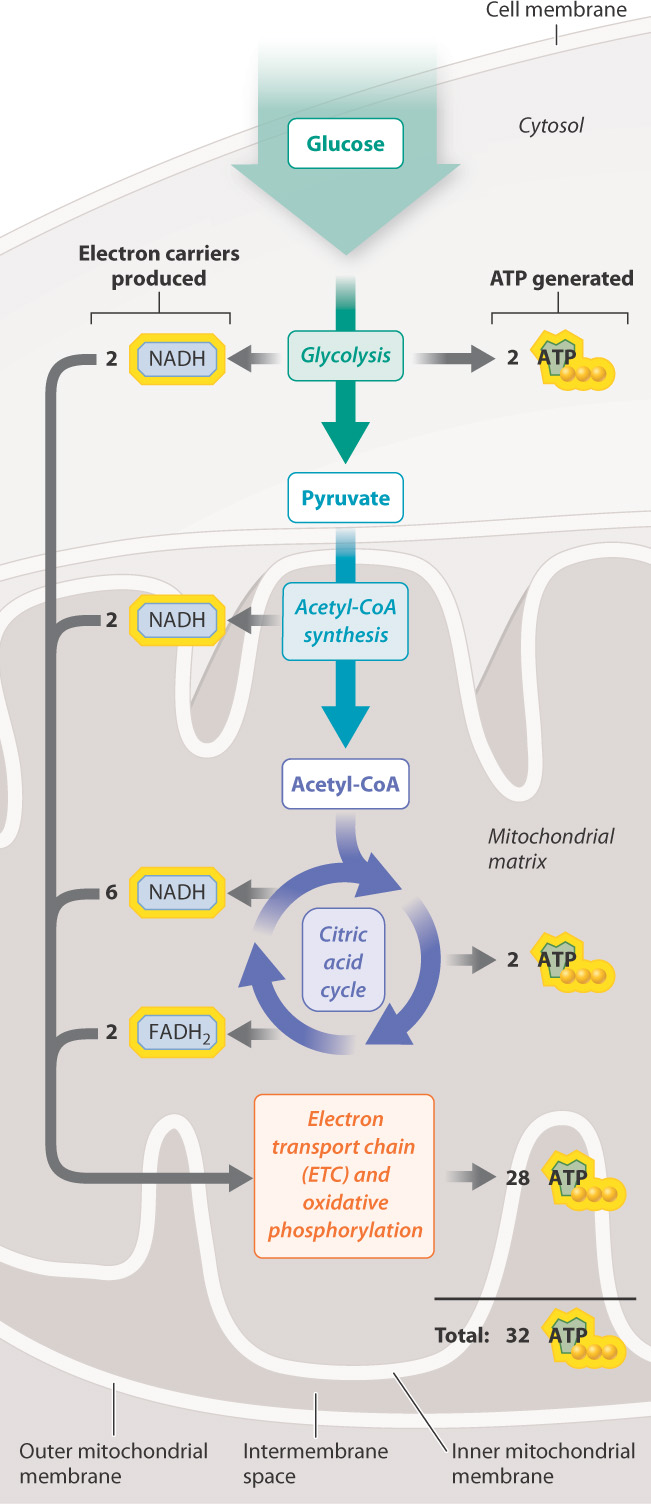
Approximately 2.5 molecules of ATP are produced for each NADH that donates electrons to the chain and 1.5 molecules of ATP for each FADH2. Therefore, overall, the complete oxidation of glucose yields about 32 molecules of ATP from glycolysis, acetyl-CoA synthesis, the citric acid cycle, and oxidative phosphorylation (Table 7.1). The energy held in the bonds of glucose is now stored in a molecule that can be readily used by cells.
It is worth taking a moment to follow the flow of energy in cellular respiration, illustrated in its full form in Fig. 7.12. We began with glucose and noted that it held chemical potential energy in its covalent bonds. This energy is released slowly in a series of reactions and captured in chemical form. Some of these reactions generate ATP directly by substrate-level phosphorylation. Others are redox reactions that transfer energy to the electron carriers NADH and FADH2. These electron carriers donate electrons to the electron transport chain, which uses the energy stored in the electron carriers to pump protons across the inner membrane of the mitochondria. In other words, the energy of the electron carriers is transformed into energy stored in a proton electrochemical gradient. ATP synthase then converts the energy of the proton gradient to rotational energy, which drives the synthesis of ATP. The cell now has a form of energy that it can use in many ways to perform work.