CHAPTER SUMMARY
7.1 CELLULAR RESPIRATION IS A SERIES OF CATABOLIC REACTIONS THAT CONVERT THE ENERGY STORED IN FUEL MOLECULES INTO ATP.
- Cellular respiration is a four-stage process that includes (1) glycolysis; (2) pyruvate oxidation and acetyl-CoA synthesis; (3) the citric acid cycle; and (4) oxidative phosphorylation.
- The complete oxidation of glucose in the presence of oxygen to make carbon dioxide and water is an oxidation-reduction reaction.
- In oxidation–reduction reactions, electrons are transferred from one molecule to another. Oxidation is the loss of electrons, and reduction is the gain of electrons.
- The chemical bonds of sugar molecules like glucose have high potential energy because the electrons that are shared by neighboring atoms are on average far from the nucleus.
- The chemical energy in glucose and other fuel molecules is released slowly in a series of chemical reactions that produce energy-storing molecules, including ATP and the electron carriers NADH and FADH2.
- Electron carriers transfer electrons to an electron transport chain, which harnesses the energy of these electrons to generate ATP.
- ATP is generated by substrate-level phosphorylation and oxidative phosphorylation in cellular respiration.
7.2 GLYCOLYSIS IS THE PARTIAL OXIDATION OF GLUCOSE AND RESULTS IN THE PRODUCTION OF PYRUVATE, A SMALL AMOUNT OF ATP, AND HIGH-ENERGY ELECTRON CARRIERS.
- Glycolysis takes place in the cytoplasm.
- Glycolysis is a series of 10 reactions in which glucose is converted to pyruvate.
- Glycolysis involves preparatory, cleavage, and payoff phases.
- For each molecule of glucose consumed during glycolysis, a net gain of two molecules of ATP and two molecules of NADH is produced.
- The synthesis of ATP in glycolysis results from the direct transfer of a phosphate group to ADP, a process called substrate-level phosphorylation.
7.3 PYRUVATE IS OXIDIZED TO ACETYL-CoA, CONNECTING GLYCOLYSIS TO THE CITRIC ACID CYCLE.
- The conversion of pyruvate to acetyl-CoA results in the production of one molecule of NADH and one molecule of carbon dioxide. Acetyl-CoA synthesis occurs in the mitochondrial matrix.
- Acetyl-CoA is the first substrate in the citric acid cycle.
7.4 THE CITRIC ACID CYCLE RESULTS IN THE COMPLETE OXIDATION OF FUEL MOLECULES AND THE GENERATION OF ATP AND HIGH-ENERGY ELECTRON CARRIERS.
- The citric acid cycle takes place in the mitochondrial matrix.
- The acetyl group of acetyl-CoA combines with oxaloacetate, followed by a series of decarboxylation and redox reactions that regenerate oxaloacetate.
- A complete turn of the citric acid cycle results in the production of one molecule of GTP (which is converted to ATP), three molecules of NADH, and one molecule of FADH2.
- Citric acid cycle intermediates are starting points for the synthesis of many different organic molecules.
7.5 THE ELECTRON TRANSPORT CHAIN TRANSFERS ELECTRONS FROM ELECTRON CARRIERS TO OXYGEN, USING THE ENERGY TO PUMP PROTONS AND SYNTHESIZE ATP BY OXIDATIVE PHOSPHORYLATION.
- NADH and FADH2 donate electrons to the electron transport chain.
- In the electron transport chain, electrons move from one redox carrier to the next.
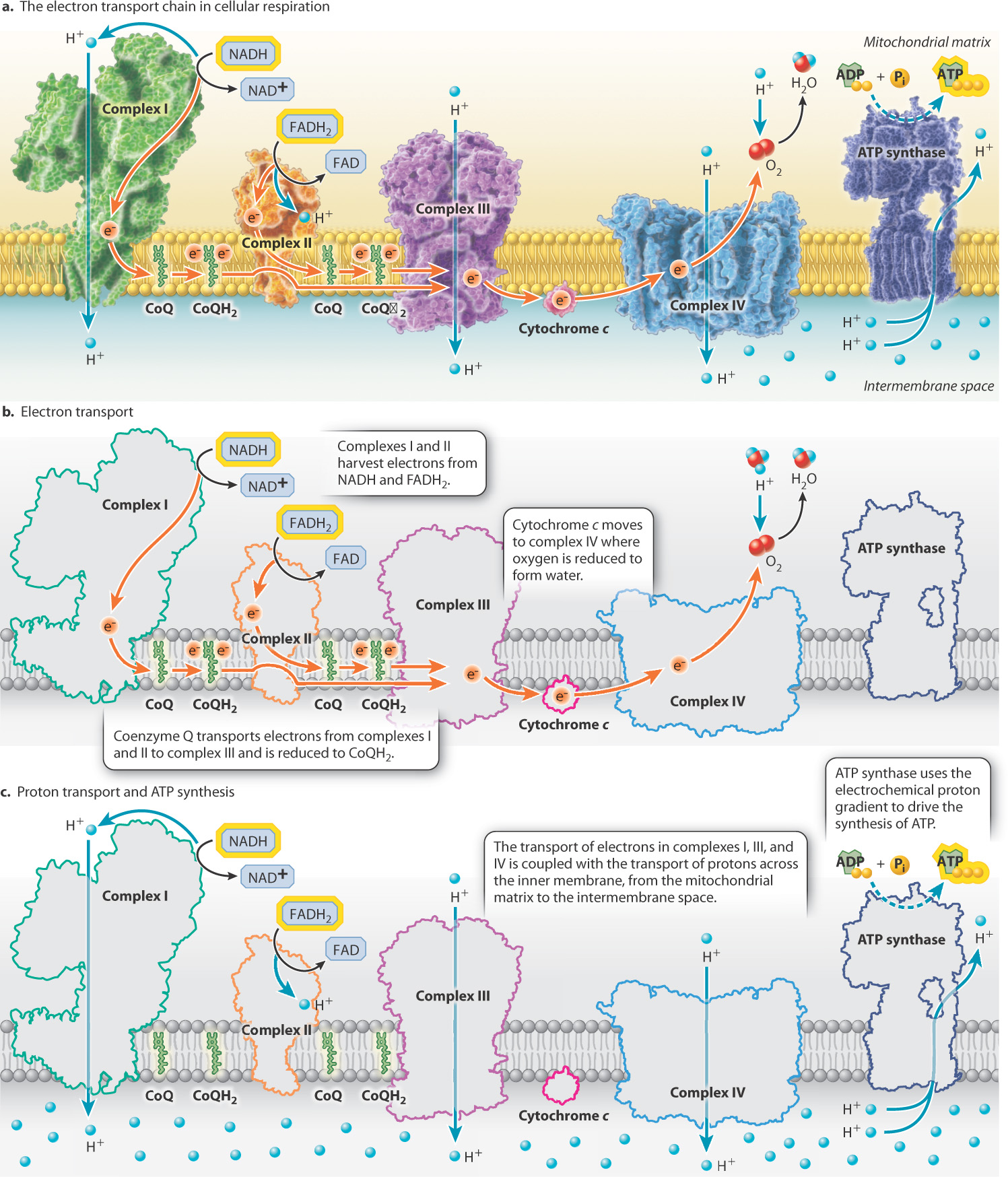
- The electron transport chain is made up of four complexes. Complexes I and II accept electrons from NADH and FADH2 respectively. The electrons are transferred from these two complexes to coenzyme Q.
- Reduced coenzyme Q transfers electrons to complex III and cytochrome c transfers electrons to complex IV. Complex IV contains oxygen, the final electron acceptor.
- The transfer of electrons through the electron transport chain is coupled with the movement of protons across the inner mitochondrial membrane into the intermembrane space.
- The buildup of protons in the intermembrane space results in a proton electrochemical gradient, which stores potential energy.
- The movement of protons back into the mitochondrial matrix through the Fo subunit of ATP synthase is coupled with the formation of ATP, a reaction catalyzed by the F1 subunit of ATP synthase.
7.6 GLUCOSE CAN BE BROKEN DOWN IN THE ABSENCE OF OXYGEN BY FERMENTATION, PRODUCING A MODEST AMOUNT OF ENERGY IN THE FORM OF ATP.
- Pyruvate, the end product of glycolysis, is processed differently in the presence and the absence of oxygen.
- In the absence of oxygen, pyruvate enters one of several fermentation pathways.
- In lactic acid fermentation, pyruvate is reduced to lactic acid.
- In ethanol fermentation, pyruvate is converted to acetaldehyde, which is reduced to ethanol.
- During fermentation, NADH is oxidized to NAD1, allowing glycolysis to proceed.
- Glycolysis and fermentation are ancient biochemical pathways and were likely used in the common ancestor of all organisms living today.
7.7 METABOLIC PATHWAYS ARE INTEGRATED, ALLOWING CONTROL OF THE ENERGY LEVEL OF CELLS.
- Excess glucose is polymerized and stored in molecules called glycogen (in animals) and starch (in plants).
- Other monosaccharides derived from the digestion of dietary carbohydrates are converted into intermediates of glycolysis.
- Fatty acids contained in triacylglycerols are an important form of energy storage in cells. The breakdown of fatty acids is called b-oxidation.
- Phosphofructokinase-1 controls a key step in glycolysis. It has many allosteric activators, including ADP and AMP, and allosteric inhibitors, including ATP and citrate.
- The ATP in muscle cells used to power exercise is generated by lactic acid fermentation, aerobic respiration, and b-oxidation.
Self-Assessment Question 1
Name and describe the four major stages of cellular respiration.
Show Model Answer
Model Answer:
Cellular respiration is a series of chemical reactions that convert the energy stored in fuel molecules into a chemical form that can readily be used by cells. Cellular respiration occurs in four main stages: (1) Glycolysis: glucose, fatty acids or amino acids are partially broken down and a modest amount of energy (in the form of ATP) is released. (2) Acetyl-CoA synthesis: pyruvate (the breakdown product of glucose from stage 1) is converted to acetyl-coenzyme A, and carbon dioxide is produced. (3) Citric acid cycle: acetyl-CoA is broken down and carbon dioxide and ATP are released. (4) Oxidative phosphorylation: in these reactions, electron carriers generated in stages 1–3 donate their high-energy electrons to an electron-transport chain (or a respiratory chain). This chain transfers electrons along a series of membrane-associated proteins to a final electron acceptor and harnesses the energy of the electrons to produce a large amount of ATP. In aerobic respiration, oxygen is the final electron acceptor, so it is consumed and water is produced.
Self-Assessment Question 2
Explain what an oxidation–reduction reaction is and why the breakdown of glucose in the presence of oxygen to produce carbon dioxide and water is an example of an oxidation–reduction reaction.
Show Model Answer
Model Answer:
Oxidation-reduction reactions are used to store or release chemical energy. Oxidation is the loss of electrons and reduction is the gain of electrons. This gain and loss always happens in a single reaction or, in other words, electrons are transferred from one molecule to another. However, in many systems, electrons are not completely transferred between the molecules. Instead, there is a change in electron density around an atom. This is shown in the breakdown of glucose in the presence of oxygen to produce carbon dioxide and water. The carbon atoms in glucose are oxidized because they go from sharing electrons equally in the bond (C-C) to partially losing electrons in the carbon dioxide molecule (C=O). The opposite is true for oxygen, which is reduced in the same reaction. The oxygen atoms go from sharing electrons equally (O=O) to partially gaining electrons when water is formed (H-O).
Self-Assessment Question 3
Describe two ways in which ATP is generated in cellular respiration.
Show Model Answer
Model Answer:
ATP is generated by substrate-level phosphorylation and oxidative phosphorylation. In substrate-level phosphorylation, a phosphorylated organic molecule directly transfers a phosphate group to ADP. This pathway produces only a small amount of the total ATP generated in the process of cellular respiration. In contrast, most of the ATP generated in cellular respiration is produced through oxidative phosphorylation (stage 4 of the cellular respiration reactions). In these reactions, ATP is generated indirectly through the reduction of electron carriers, the transfer of high-energy electrons from electron carriers to the electron-transport chain, and the subsequent synthesis of ATP from ADP and inorganic phosphate.
Self-Assessment Question 4
Write the overall chemical equation for glycolysis, noting the starting and ending products and highlighting the energy–storing molecules that are produced.
Show Model Answer
Model Answer:
Starting product is glucose and the end product is pyruvate Energy storing molecules that are produced are ATP and NADH-. Glucose + 2NAD+ + 2ADP + 2Pi → 2 pyruvate + 2ATP + 2NADH + 2H+ + 2H2O
Self-Assessment Question 5
Describe two different metabolic pathways that pyruvate can enter.
Show Model Answer
Model Answer:
In the first pathway, pyruvate is converted to acetyl-CoA, which is the starting substrate for the citric acid cycle. During the citric acid cycle, the chemical energy in the bonds of acetyl-CoA is transferred to ATP by substrate-level phosphorylation and to the electron carriers NADH and FADH2. The second pathway is fermentation, a reaction that happens without oxygen. There are many fermentation pathways but all rely on oxidation of NADH to NAD+ when pyruvate or a derivative of pyruvate is reduced. Two major fermentation pathways are lactic acid fermentation and ethanol fermentation. In the lactic acid pathway, electrons from NADH are transferred to pyruvate to produce lactic acid and NAD+. In the ethanol fermentation pathway, pyruvate releases carbon dioxide to form acetaldehyde, and electrons from NADH are transferred to the molecule to produce ethanol and NAD+.
Self-Assessment Question 6
Name the products of the citric acid cycle.
Show Model Answer
Model Answer:
In two turns of the citric acid cycle (one for each acetyl CoA), 2 ATP, 6 NADH, and 2 FADH2 are produced. Carbon dioxide and oxaloacetate are also produced.
Self-Assessment Question 7
Describe how the movement of electrons along the electron transport chain leads to the generation of a proton gradient.
Show Model Answer
Model Answer:
The movement of electrons along the electron-transport chain in the inner mitochondrial membrane is coupled to the transfer of protons through several enzyme complexes and electron carriers. NADH donates an electron to Complex I. From there, Coenzyme Q (CoQ) picks up the electron and transfers it to either Complex II or Complex III. Complex III donates the electron to Cytochrome c, which in turn transfers it to Complex IV, which then donates it to the final electron acceptor oxygen. As the electrons pass through the complexes, protons are pumped into the intermembrane space. This creates a concentration and charge gradient, providing a source of potential energy that is then used to drive the synthesis of ATP. See Figure 7.9.
Self-Assessment Question 8
Describe how a proton gradient generates ATP.
Show Model Answer
Model Answer:
The protons accumulated in the intermembrane space cannot passively diffuse across the membrane, so they utilize a transport channel called ATP synthase. This enzyme is composed of two subunits: Fo (the channel through which protons flow) and F1 (the catalytic unit that synthesizes ATP). Proton flow through the channel causes it to rotate, which converts the energy of the proton gradient into mechanical rotational energy (kinetic energy). The rotation of the Fo subunit leads to rotation of the F1 subunit. This causes conformational changes in the F1 subunit that allow it to catalyze the synthesis of ATP from ADP and Pi.
Self-Assessment Question 9
Explain how muscle tissue generates ATP during short-term and long-term exercise.
Show Model Answer
Model Answer:
Muscle tissue generates ATP during short-term exercise by converting stored glycogen to glucose. This rapid breakdown of glucose is done anaerobically to pyruvate and lactic acid, which then feeds into the lactic acid fermentation pathway. During long-term exercise, the liver release glucose into the blood, which is taken up by muscle cells and oxidized to produce ATP. In addition, adipose tissue releases fatty acids that are also taken up by muscle cells and broken down by ß-oxidation. These processes are slower to mobilize the conversion of glucose and other molecules to energy; however, the end result is the production of more ATP than the fermentation pathway.