8.1 THE NATURAL HISTORY OF PHOTOSYNTHESIS
Carbohydrates have more energy stored in their chemical bonds than is contained in the bonds of the CO2 molecules from which they are synthesized during photosynthesis. Therefore, to build carbohydrates using CO2 requires an input of energy. In photosynthesis, this energy comes from sunlight.
8.1.1 Photosynthesis is a redox reaction.
Energy is added to molecules during carbohydrate synthesis through the transfer of high-energy electrons. It is this addition of energy and electrons that allows the incoming CO2 molecules to form the higher-energy bonds found in a carbohydrate molecule. In Chapter 7, we saw that reduction reactions are reactions in which a molecule acquires electrons and gains energy, whereas oxidation reactions are reactions in which a molecule loses electrons and releases energy. During photosynthesis, CO2 molecules are reduced to form higher-energy carbohydrate molecules (Fig. 8.1).
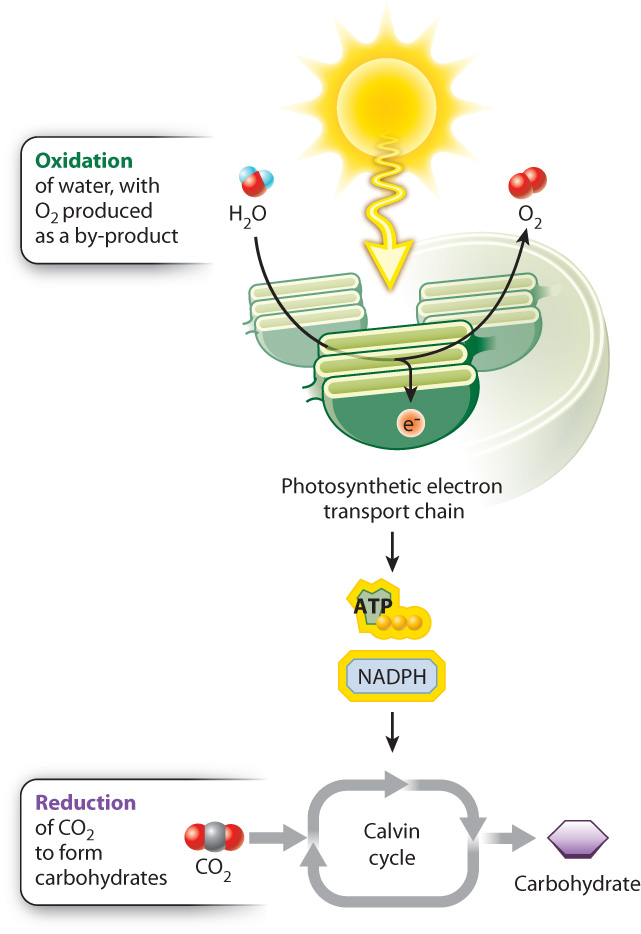
Where do the electrons used to reduce CO2 come from? These electrons can only come from the oxidation of other molecules, illustrating once again that reduction–oxidation (or redox) reactions always come in pairs. In photosynthesis carried out by plants and many algae, the ultimate electron donor is water. However, as we will see in Chapter 26, photosynthetic bacteria can use a variety of other electron donors. The oxidation of water results in the production of electrons, protons, and O2. Thus, oxygen is formed in photosynthesis as a by-product of water’s role as a source of electrons. We can demonstrate that water is the source of the oxygen released during photosynthesis using isotopes, molecules that can be distinguished on the basis of their molecular mass (Fig. 8.2).
FIG. 8.2Does the oxygen released by photosynthesis come from H₂O or CO₂?
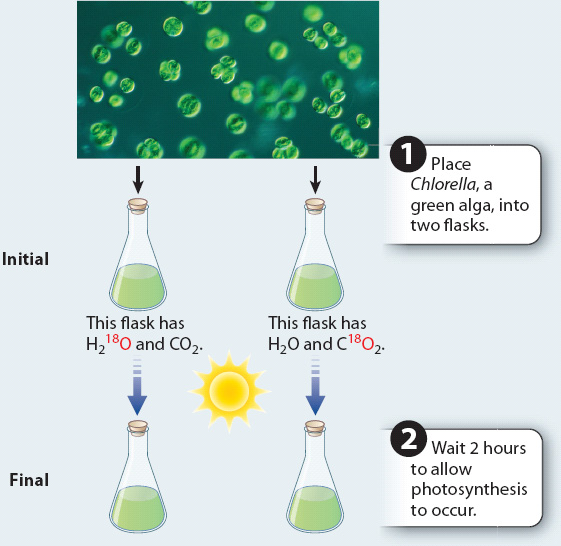
BACKGROUND The reactants in photosynthesis are water and carbon dioxide. Therefore, it is unclear where the oxygen that is produced in the reaction comes from.
METHOD Most of the oxygen in the atmosphere is 16O, with 8 protons and 8 neutrons. A small amount (0.2%) is 18O, with 8 protons and 10 neutrons. 16O and 18O are stable isotopes. The relative abundance of molecules containing 16O versus 18O can be measured using a mass spectrometer. H2O and CO2 containing a high percentage of 18O can be used to determine whether the oxygen produced in photosynthesis comes from water or carbon dioxide.
EXPERIMENT
RESULTS
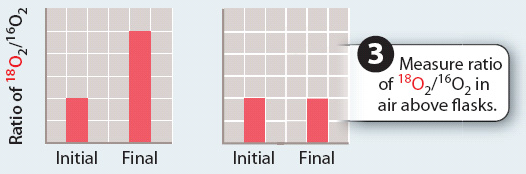
CONCLUSION The ratio of 18O2/16O2 only increases when water contains 18O, but not when carbon dioxide contains 18O. This finding indicates that the oxygen produced in photosynthesis comes from water, not carbon dioxide.
FOLLOW-UP WORK Carbon also has several isotopes, and their measurement has been used to determine the source of increased CO2 in the atmosphere today (Chapter 25).
SOURCE Adapted from Ruben, S., M. Randall, M. Kamen, and J. L. Hyde. 1941. “Heavy Oxygen (O18) as a Tracer in the Study of Photosynthesis.” Journal of the American Chemical Society. 63:877–879.
Overall, then, the equation for photosynthesis can be described as follows:
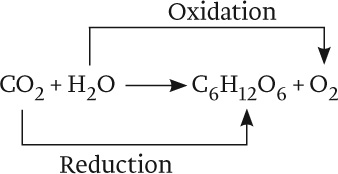
The oxidation of water is linked with the reduction of CO2 through a series of redox reactions in which electrons are passed from one compound to another. This series of reactions constitutes the photosynthetic electron transport chain. The process begins with the absorption of light by protein–pigment complexes known as photosystems. Photosystems use absorbed light energy to drive redox reactions and thereby set the photosynthetic electron transport chain in motion. In turn, the movement of electrons through this transport chain is used to drive the synthesis of ATP and NADPH. And finally, ATP and NADPH are the energy sources needed to synthesize carbohydrates using CO2 in a process called the Calvin cycle (see Fig. 8.1).
Each year, photosynthesis removes more than 100 billion metric tons of carbon from the atmosphere, while incorporating more than 100 terawatts (a terawatt is 1012 watts) of solar energy into chemical bonds. To put these quantities in perspective, the energy captured by photosynthesis in a year is 10 times the energy of all the oil and coal used worldwide in 2006. Fossil fuels are themselves the legacy of ancient photosynthesis: Oil has its origin in the bodies of marine phytoplankton and the organisms that graze on them, while coal represents the geologic remains of terrestrial (land) plants. Thus, one motivation to understand photosynthesis is the sheer magnitude and importance of this process for life on Earth. Before exploring the details of how photosynthesis actually occurs, let’s look at what types of organisms are photosynthetic, where they live, how they have evolved to be photosynthetic, and what structural components are needed to allow cells to capture energy in this remarkable way.
Quick Check 1
If you want to produce carbohydrates containing the heavy oxygen (18O) isotope, should you water your plants with H218O or inject C18O2 into the air?
8.1.2 Photosynthesis is widely distributed.
Photosynthesis occurs among prokaryotic as well as eukaryotic organisms, on land as well as in the sea. Approximately 50% of global photosynthesis is carried out by terrestrial organisms, with the other half taking place in the ocean. The majority of photosynthetic organisms in marine environments are unicellular. About half of oceanic photosynthesis is carried out by phytoplankton (single-celled marine eukaryotes), while the other half is carried out by cyanobacteria (photosynthetic bacteria). On land, photosynthesis is dominated by multicellular plants.
Photosynthesis takes place almost everywhere sunlight is available to serve as a source of energy. In the ocean, photosynthesis occurs in the surface layer about 100 m deep, called the photic zone, through which enough sunlight penetrates to enable photosynthesis. On land, photosynthesis occurs most readily in environments that are both moist and warm. However, photosynthetic organisms have evolved adaptations that allow them to tolerate a wide range of environmental conditions, like those illustrated in Fig. 8.3. In very dry regions, a combination of photosynthetic bacteria and unicellular algae forms an easily disturbed layer on the surface of the soil known as desert crust. Photosynthetic bacteria are also found in the hot springs of Yellowstone National Park at temperatures up to 75˚C. At the other extreme, unicellular eukaryotic algae can grow on the surfaces of glaciers, causing the surface of the snow to appear red. In section 8.4, we discuss why photosynthetic organisms growing in such extreme environments often appear red or yellow rather than green.

8.1.3 The evolutionary history of photosynthesis includes both horizontal gene transfer and endosymbiosis.
The most ancient forms of photosynthesis have simple photosynthetic electron transport chains with only a single photosystem. However, a single photosystem cannot capture enough energy to pull electrons from (oxidize) water and then use them to reduce CO2. Thus, photosynthetic organisms with a single photosystem must use more easily oxidized compounds, such as H2S, as electron donors. These organisms are limited to environments where the electron-donor molecules are abundant. Because these organisms do not use water as an electron donor, they do not produce O2 during photosynthesis (Chapter 26).
A major event in the history of life was the evolution of photosynthetic electron transport chains that could bridge the energy difference between the oxidation of water and the reduction of CO2. Water is abundant, but it is extremely difficult to pull electrons from water. The first organisms to accomplish this feat were the cyanobacteria. These photosynthetic bacteria incorporated two different photosystems into a single photosynthetic electron transport chain. Having two photosystems allows the energy level of the electrons moving through the photosynthetic electron chain to be increased in two steps, much as a series of locks serves to raise the elevation of boats traveling through a canal. The addition of light energy in the first step allows electrons to be pulled from a water molecule, while the addition of light energy in the second step raises the energy level of these electrons so that they can be used to reduce CO2. Stripping electrons from water results in the release of oxygen. Indeed, all the oxygen in Earth’s atmosphere results from photosynthesis by organisms containing two photosystems.
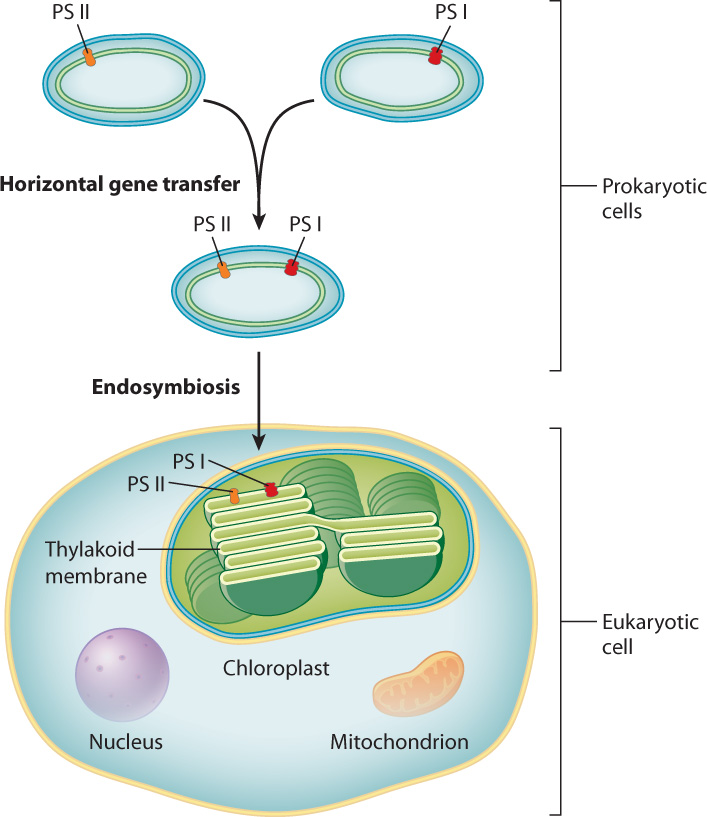
Each of the two photosystems present in cyanobacteria has a high degree of similarity to the single photosystem found in other lineages of photosynthetic bacteria. The structural similarity is so great as to make it highly unlikely that the two photosystems in cyanobacteria evolved independently. An alternative hypothesis is that the genetic material associated with one photosystem was transferred to a bacterium with the other photosystem, resulting in a single bacterium with the genetic material to produce both types of photosystems (Fig. 8.4). The transfer of genetic material between organisms that are not parent and offspring is called horizontal gene transfer, and it is common among prokaryotes, where it provides an important means of producing genetic diversity. The mechanisms of horizontal gene transfer are discussed more fully in Chapter 26.
Photosynthesis is hypothesized to have gained a foothold among eukaryotic organisms when a free-living cyanobacterium was engulfed by a eukaryotic cell (Fig. 8.4). Over time, the engulfed cyanobacterium lost its ability to survive outside of its host cell and evolved into the chloroplast, the organelle in eukaryotic cells that carries out photosynthesis. The process in which one cell takes up residence inside of another cell is called endosymbiosis. Therefore, the idea that chloroplasts and mitochondria (Chapter 7) arose in this way is called the endosymbiotic hypothesis. It is discussed in Chapter 27.
8.1.4 The photosynthetic electron transport chain takes place on specialized membranes.
The ability to absorb sunlight and convert it into chemical forms allows photosynthetic organisms to produce their own food. However, high-energy electrons moving through the photosynthetic electron transport chain have the potential to damage the cell. To prevent damage, the movement of electrons through the photosynthetic electron transport chain is constrained: The electrons follow a defined network of pathways through large protein complexes embedded in specialized “photosynthetic” membranes. These protein complexes provide a scaffold along which the pigments and other elements of the photosynthetic electron transport chain are arrayed. In fact, the movement of electrons through the photosynthetic electron transport chain is not controlled by the specificity of individual enzymes. Instead, it depends upon the spatial arrangement of the sites where the redox reactions take place within and between these major protein complexes.
Chloroplasts are enclosed by a double membrane. Filling much of the center of the chloroplast is a third, highly folded, membrane known as the thylakoid membrane (Fig. 8.5). The photosynthetic electron transport chain is located on the thylakoid membrane.
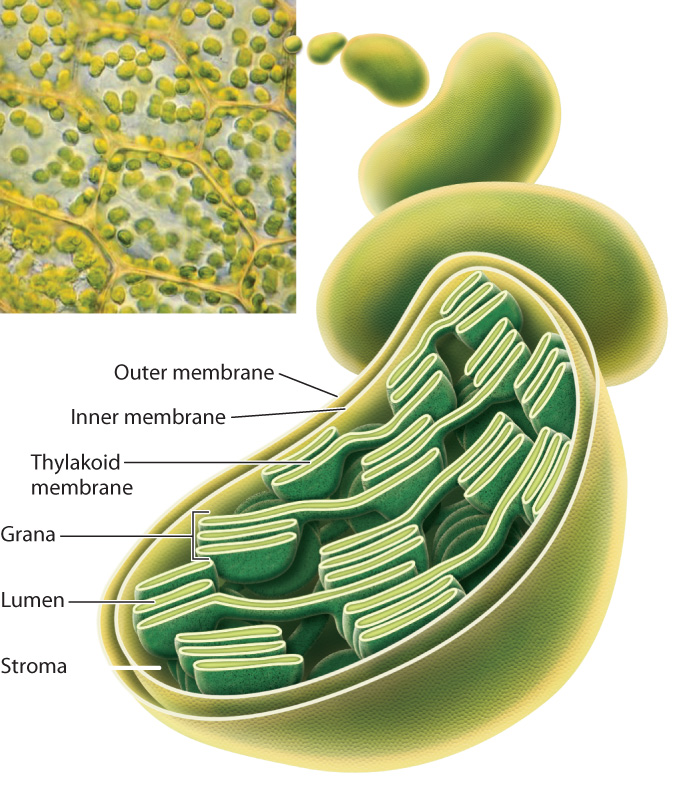
The name “thylakoid” is derived from thylakois, the Greek word for “sac.” Thylakoid membranes form structures that resemble flattened sacs, which are grouped into structures called grana (singular, granum) that look like stacks of interlinked pancakes. Grana are connected to one another by membrane bridges so that the thylakoid forms a single continuous membrane. Thus, while it may look as though the individual grana are independent from one another, in fact the thylakoid membrane encloses a common fluid-filled interior compartment called the lumen. The region surrounding the thylakoid membrane is called the stroma. Carbohydrate synthesis takes place in the stroma, whereas sunlight is captured and transformed via the photosynthetic electron transport chain on the thylakoid membrane.
The outer membrane of the chloroplast double membrane is thought to have originated from the plasma membrane of the ancestral eukaryotic cell, which surrounded the ancestral cyanobacterium as it was being engulfed. The inner chloroplast membrane is thought to correspond to the plasma membrane of the ancestral free-living cyanobacterium. The thylakoid membrane then corresponds to the internal photosynthetic membrane found in cyanobacteria. Finally, the stroma corresponds to the cytoplasm of the ancestral cyanobacterium.
A photosynthetic cell can have more than 100 chloroplasts. Photosynthetic cells also contain mitochondria. Although photosynthetic organisms are correctly described as autotrophs because they can form carbohydrates from CO2, they also require mitochondrial respiration, in which carbohydrates are broken down to generate ATP to meet the cell’s energy requirements (Chapter 7). Cellular respiration is therefore one of several features that heterotrophic organisms like ourselves share with photosynthetic organisms.
We now consider the underlying biochemistry of photosynthesis. Though in this chapter we focus on photosynthesis as carried out by eukaryotic cells, there is a remarkable diversity in the way photosynthesis is carried out in bacteria. We discuss this diversity in Chapter 26. Because carbohydrates are the major product of photosynthesis, we first examine the Calvin cycle, the biochemical pathway used in photosynthesis to synthesize carbohydrates from CO2. Once we understand what energy forms are needed to drive this autotrophic pathway, we turn our attention to how energy is captured from sunlight. Finally, we examine some of the challenges of coordinating these two metabolic stages and consider ways in which photosynthetic organisms cope with the inherent biochemical challenges of photosynthesis.