10.3 CELLULAR MOVEMENT
In addition to providing structure and support, some cytoskeletal elements also allow cellular movement. We can think of cellular movement in three different ways. First, there is the movement of the cell itself, and many cells are capable of moving great distances on their own. The second kind of movement is the change in shape of a cell, as when a contracting muscle cell shortens. Changes in cell shape are also common in embryos and are an essential part of early development. The third kind of cellular movement is the movement of molecules and organelles within a cell. For example, melanocytes in the epidermis transport their packets of melanin along their branching dendrites to deliver them to neighboring keratinocytes (see Fig. 10.2). Similarly, in neurons, neurotransmitters are transported from the cell body to the end of the axon, where they are stored until they are needed. In this section, we discuss the molecular basis for these kinds of cellular movement.
10.3.1 Motor proteins associate with microtubules and microfilaments to cause movement.
A motor is a device that imparts motion. We saw that microtubules and microfilaments have some capacity to move by polymerization and depolymerization, but on their own their capacity for movement is fairly limited. However, when joined by small accessory proteins called motor proteins, microtubules and microfilaments are capable of causing amazing movements.
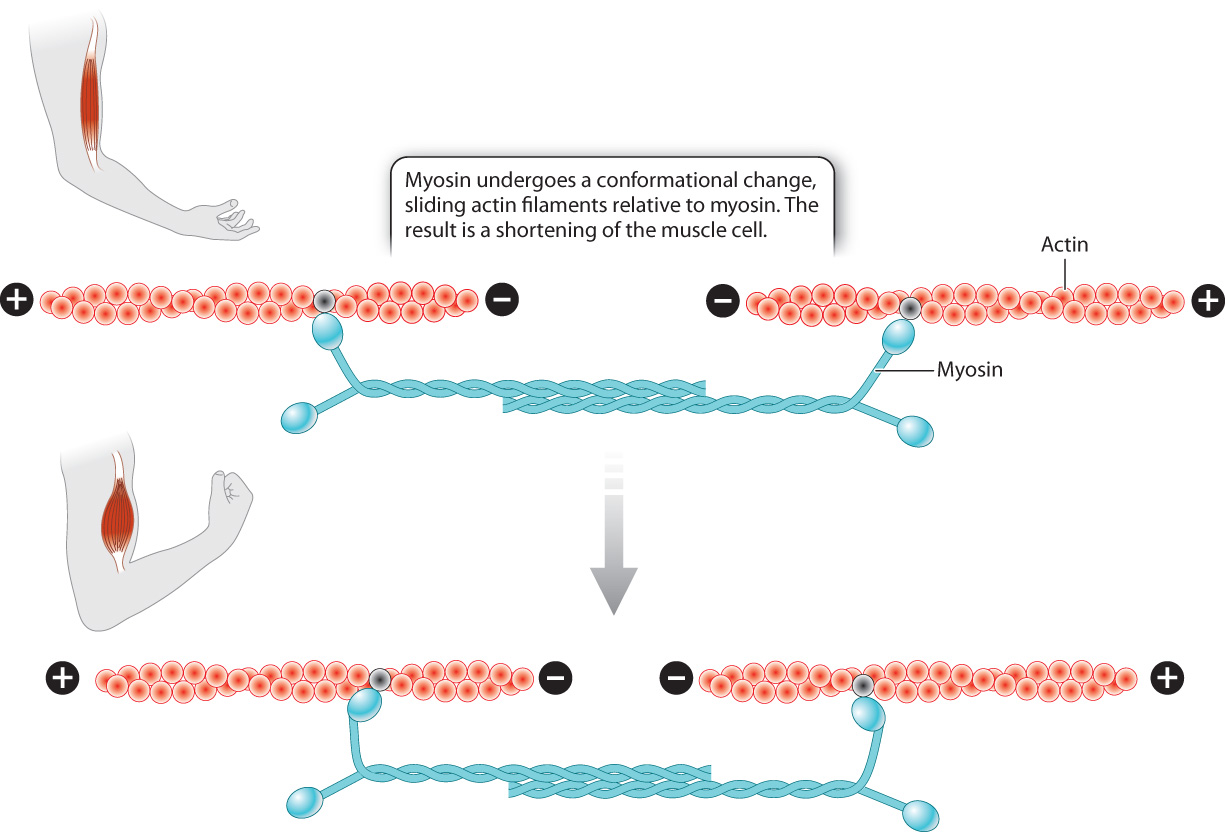
First let’s consider how these motor proteins contribute to a change in cell shape. Motor proteins cause muscle contraction by moving the actin microfilaments inside muscle cells. The cytoplasm of muscle cells shown in Fig. 10.1c is packed with actin microfilaments that are anchored to the ends of the cell. Myosin, a motor protein found in muscle cells, binds to actin and undergoes a conformational change. As a result, the actin microfilaments slide relative to myosin, causing the cell to shorten, or contract (Fig. 10.10). The energy required for the conformational change in myosin and the movement of actin microfilaments comes from the energy stored in ATP and is discussed in Chapter 37.
In other cells, myosin attached to various types of cellular cargo, such as transport vesicles, works by a similar mechanism to move materials from one part of the cell to another, using microfilaments as tracks (Fig. 10.11a).
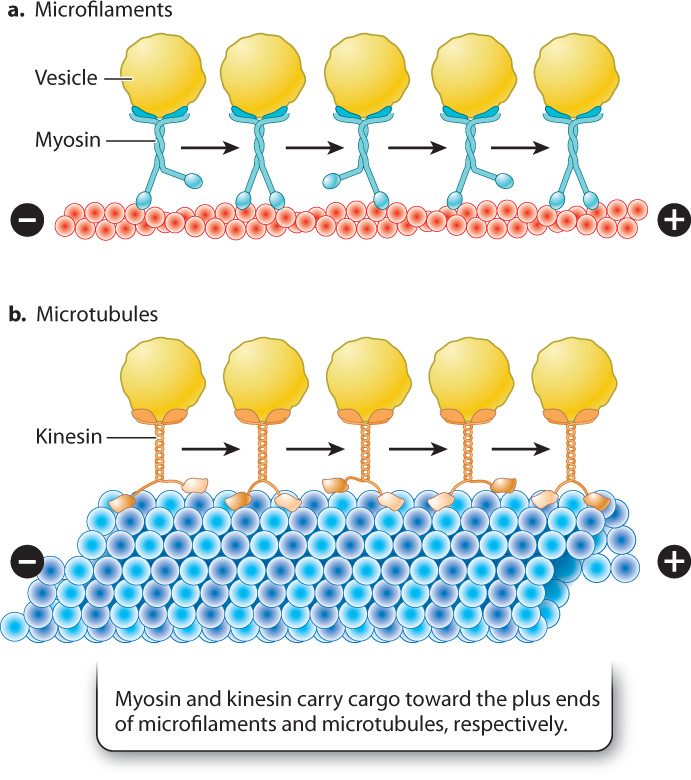
Microtubules also function as tracks for transport within the cell. Transport along microtubules takes place in a similar fashion to transport along microfilaments, except that the two motor proteins that transport cargo are kinesin and dynein. Kinesin is similar in structure to myosin and transports cargo toward the plus end of microtubules at the periphery of the cell (Fig. 10.11b). By contrast, dynein carries its load away from the plasma membrane toward the minus end. As with myosin, movement along microtubules by kinesin and dynein is driven by conformational changes in the motor proteins and is powered by energy harvested from ATP.
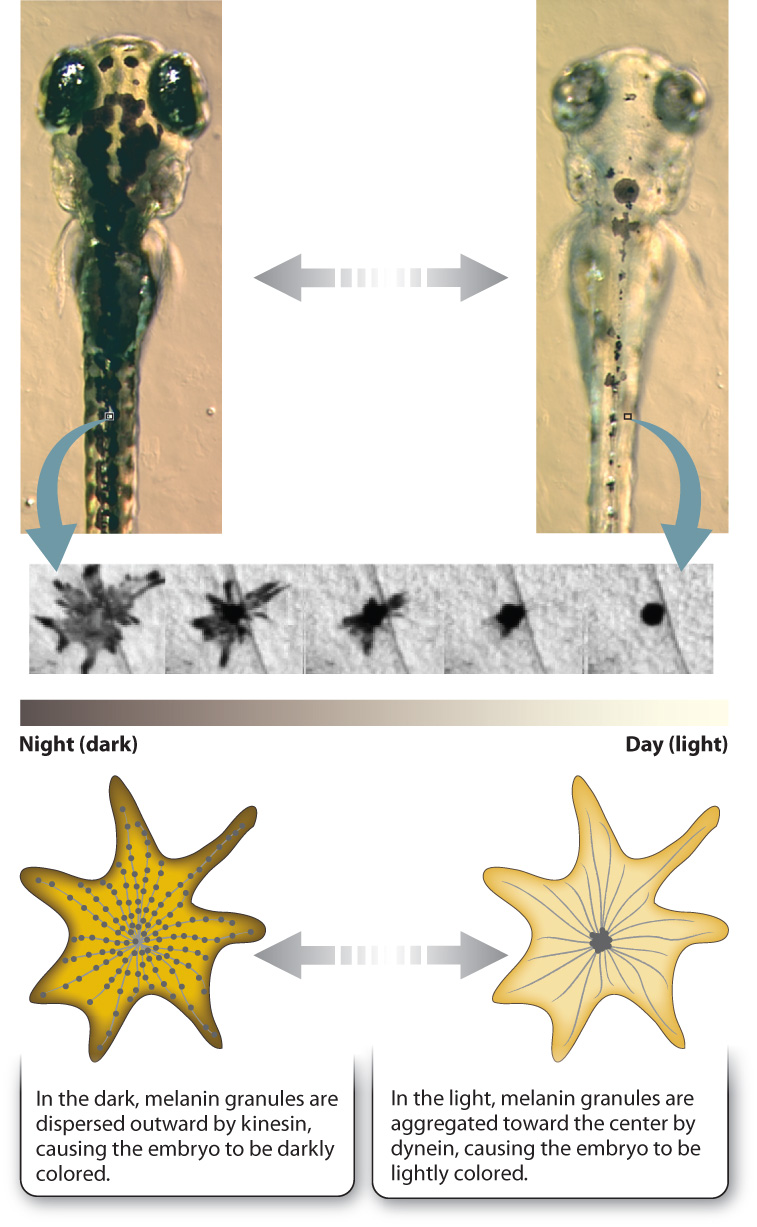
Let’s look at an especially striking example of this system at work in the specialized skin cells called melanophores present in some vertebrates. Melanophores are similar to the melanocytes in our skin, but rather than hand off their melanin to other cells, as in humans, melanophores keep their pigment granules and move them around the cell in response to hormones or neuronal signals. This redistribution of melanin within the cell allows animals such as fish or amphibian embryos to change color. For example, at night the melanin granules in the skin of a zebrafish embryo are dispersed throughout the melanophores, making it darkly colored. As morning comes and the day brightens, the pigment granules are aggregated at the center of the cell around the centrosome, causing the embryo’s color to lighten (Fig. 10.12). The melanin granules in the melanophores move back and forth along microtubules, transported by kinesin and dynein. Kinesin moves the granules out toward the plus end of the microtubule during dispersal, and dynein moves them back toward the minus end during aggregation. The daytime and nighttime camouflage provided by this mechanism of color change keeps young, developing organisms from being spotted by hungry predators lurking below.
Quick Check 1
Would a defect in dynein or kinesin cause a zebrafish embryo to remain darkly colored after daybreak?
10.3.2 Organelles with special arrangements of microtubules propel cells through the environment.
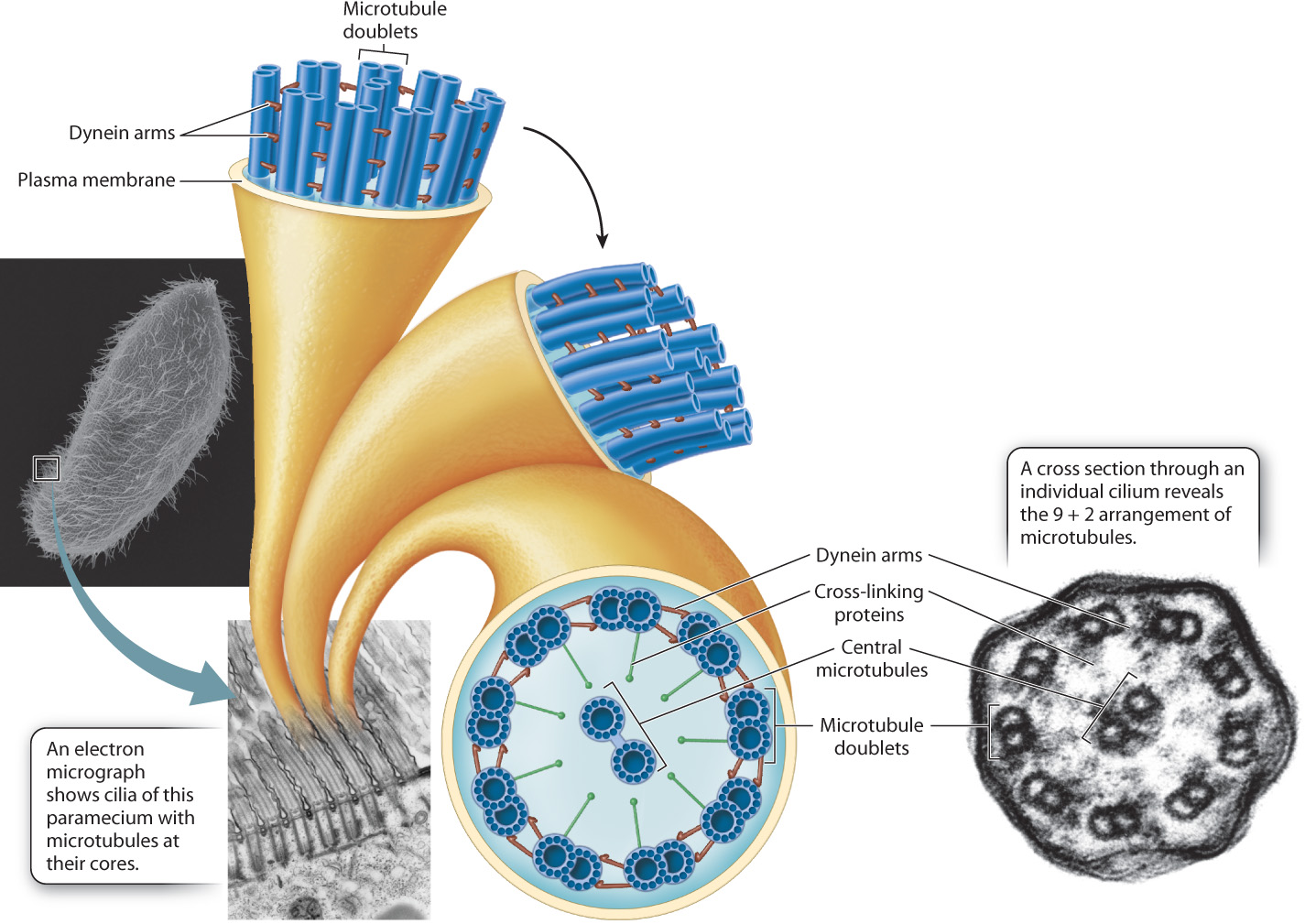
Many single-celled eukaryotic organisms that live in aquatic environments propel themselves through the water by means of the motion of short cilia or long flagella (see Fig. 10.4). These organelles are fiberlike extensions of the plasma membrane that have microtubules extending their entire length. Some cells of multicellular organisms also have cilia or flagella. For example, the sperm cells of algae, some plants, and many animals are propelled by one or more flagella. Epithelial cells in a number of animal tissues, such as the lining of the trachea and the upper respiratory tract, have cilia that move substances along the surface of the cell layer.
The microtubules in cilia and flagella are distributed in a characteristic “9 + 2” arrangement, that is, nine pairs of microtubules are located around the periphery of these organelles and two microtubules are at the center (Fig. 10.13). The outer microtubules are connected to the center pair by cross-linking proteins and to their neighbors by dynein molecules. Energy harvested by the hydrolysis of ATP powers the motion of cilia and flagella. Dynein undergoes a conformational change that causes the pairs of microtubules to slide past each other. The sliding of the microtubules results in a whiplike motion in the case of flagella and an oarlike rowing motion in the case of cilia.
10.3.3 Actin polymerization moves cells forward.
Both single-celled amoebas foraging for food on the bottom of a pond and mammalian white blood cells chasing down foreign bacteria rely on actin polymerization to get from one place to another. In fact, many cells move through their environment by crawling across a substrate or by squeezing between other cells and burrowing through connective tissues, rather than by using cilia or flagella. This type of movement relies on microfilaments. Commonly, new microfilaments are assembled and extended at one end of the cell and existing microfilaments are pulled together at the other end (Fig. 10.14). The polymerization of actin into a microfilament exerts a considerable amount of force, enough to push the plasma membrane out into a thin, sheetlike structure called a lamellipodium, from the Greek for “thin-layered foot.” New points of adhesion are established between the lamellipodium and the substrate.
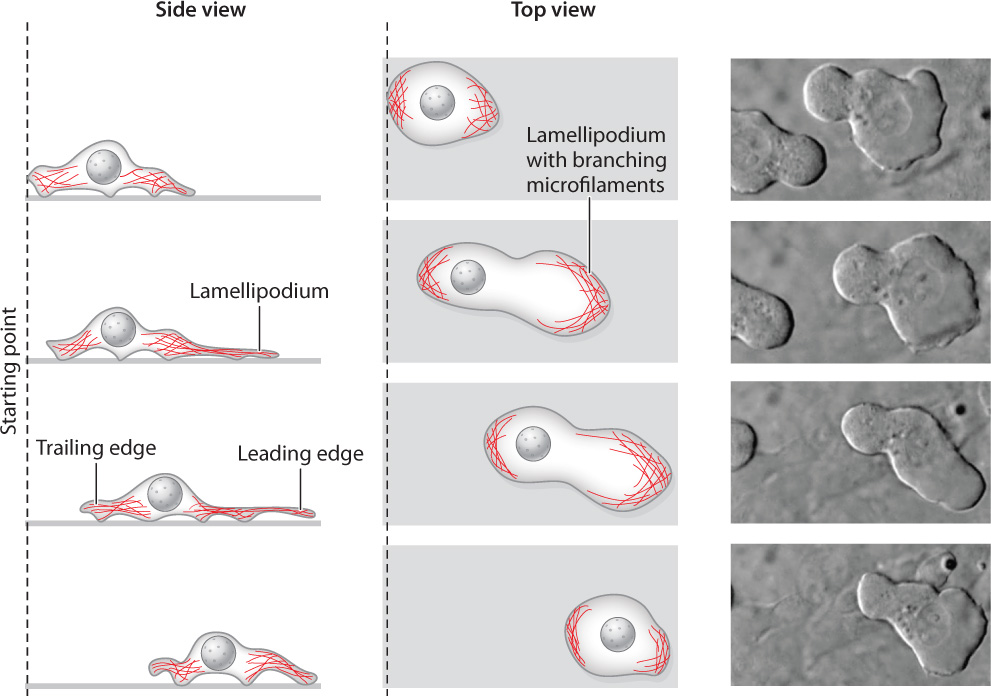
Meanwhile, bundles of actin filaments at the other end of the cell contract by the interactions of myosin and actin, squeezing the cytoplasm and its contents forward toward the lamellipodium. The edge of the cell where the lamellipodium forms is called the leading edge, and the end where contraction takes place is called the trailing edge. Repeated cycles of actin polymerization at the leading edge and microfilament contraction at the trailing edge propel the cell forward. Cells are able to migrate considerable distances by this method.
Cells do not have permanent “front” and “back” ends. Instead, the locations of the leading and trailing edges can change in an instant. This flexibility allows a migrating cell to change its direction of movement, toward a source of nutrients, for example. The formation of a lamellipodium is triggered when a cell-surface receptor binds to a nutrient molecule or other substance, so the lamellipodium forms in the region of the plasma membrane where the signal is strongest. In this way, cells can follow the concentration gradient of a nutrient to its source.