10.5 THE EXTRACELLULAR MATRIX
So far, we have looked at how the cytoskeleton maintains the shape of cells. We have also seen how the stable association of animal cells with one another and with the extracellular matrix is made possible by cellular junctions, and that these junctions are reinforced by the cytoskeleton. As important as the cytoskeleton and cellular junctions are to the structure of cells and tissues, it is the extracellular matrix that provides the molecular framework that ultimately determines the structural architecture of plants and animals.
The extracellular matrix is synthesized, secreted, and modified by many different kinds of cell. It is an insoluble meshwork composed of proteins and polysaccharides. There are many different forms of extracellular matrix, which differ in the amount, type, and organization of the proteins and polysaccharides that make them up.
In plants, the extracellular matrix takes the form of the cell wall, which provides the support needed by individual cells and results in turgor pressure (Chapter 5). Collectively, the interconnected cell walls of a plant act as an internal framework to support the entire organism. In animals, the extracellular matrix is present in most tissues and is especially abundant in connective tissue, where it provides support and protection, and in the basal lamina found underneath all epithelial tissue. In both plants and animals, the extracellular matrix not only contributes structural support but also provides informational cues that determine the activity of the cells that are in contact with it.
10.5.1 The extracellular matrix of plants is the cell wall.
The paper we write on, the cotton fibers in the clothes we wear, the wood in the chairs we sit on are, in fact, the extracellular matrix of plants. In plants, the extracellular matrix forms the cell wall, and the main component of the plant cell wall is the polysaccharide cellulose discussed in Chapter 2. Its presence in the cell wall of every plant makes cellulose the most widespread organic macromolecule on Earth.
The plant cell wall represents possibly one of the most complex examples of an extracellular matrix. It is certainly one of the most diverse in the functions it performs. Cell walls maintain the shape and turgor pressure of plant cells, allow plant cells to grow, and act as a barrier that prevents foreign materials and pathogens from reaching the plasma membrane. In many plants, cell walls collectively serve as a skeletal support structure for the entire plant.
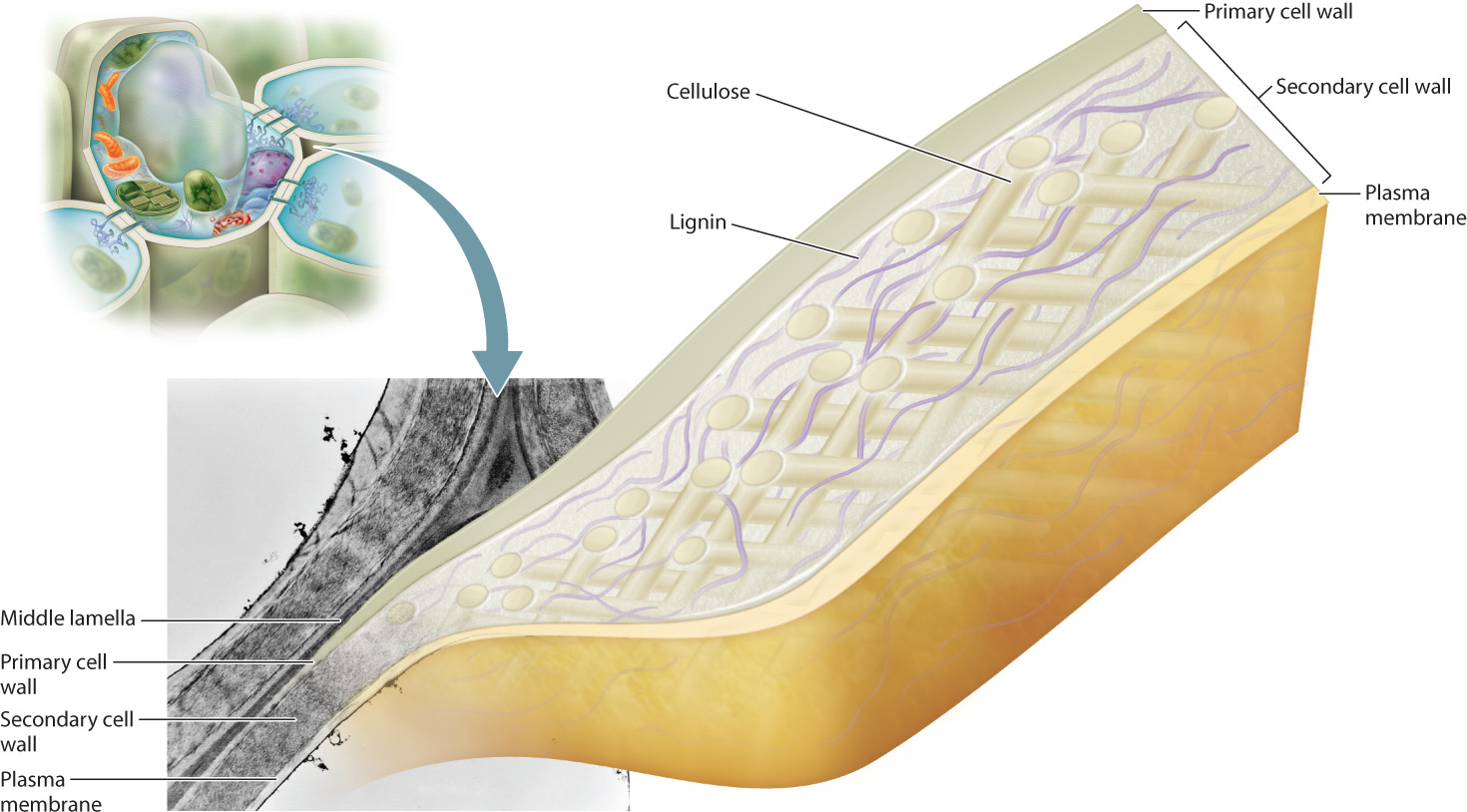
The plant cell wall is composed of as many as three layers: the outermost middle lamella, the primary cell wall, and, closest to the plasma membrane, the secondary cell wall (Fig. 10.18). The middle lamella is made first, and is synthesized during the late stages of cell division. It is made of a gluelike complex carbohydrate, and it is the main mechanism by which plant cells adhere to one another. The primary cell wall is formed next and consists mainly of cellulose fibers, but it also contains a number of other molecules, including pectin and several proteins. The primary cell wall is laid down while the cells are still growing. It is assembled by enzymes on the surface of the cell and remains thin and flexible. Once cell growth has stopped, the secondary cell wall is constructed in many, but not all, plant cells. It also is made largely of cellulose fibers but in addition contains a substance called lignin. Lignin hardens the cell wall and makes it water resistant. In woody plants, the cell wall can be up to 25% lignin. The rigid secondary cell wall permits the growth of woody plants to tremendous heights. Giant sequoia trees grow to more than 300 feet and are supported entirely by the lignin-reinforced cellulose fibers of the interconnected cell walls.
For a plant cell to grow, the cell wall must accommodate an increase in cell volume and surface area. Even though the matrix of cellulose and other polysaccharides of the primary cell wall is relatively flexible compared to the lignin-containing secondary cell wall, it is still very strong. When a plant cell grows, additional cell wall components must be synthesized to expand the area of the wall. Unlike the extracellular matrix components that are secreted by animal cells, the cellulose polymer is assembled outside the cell, on the extracellular surface of the plasma membrane. Both the glucose monomers that form the polymer and the enzymes that attach them are delivered to the cell surface by arrays of microtubules. Here is yet another example of how the cytoskeleton plays an indispensable role in regulating the shape of cells.
10.5.2 The extracellular matrix is abundant in connective tissues of animals.
The extracellular matrix of animals, like that of plants, is secreted by cells and is a mixture of proteins and polysaccharides. The animal extracellular matrix is composed of large fibrous proteins, including collagen, elastin, and laminin, which impart tremendous tensile strength. These fibrous proteins are embedded in a gel-like polysaccharide matrix. The matrix is negatively charged, attracting positively charged ions and water molecules that provide protection against compression and other physical stress.
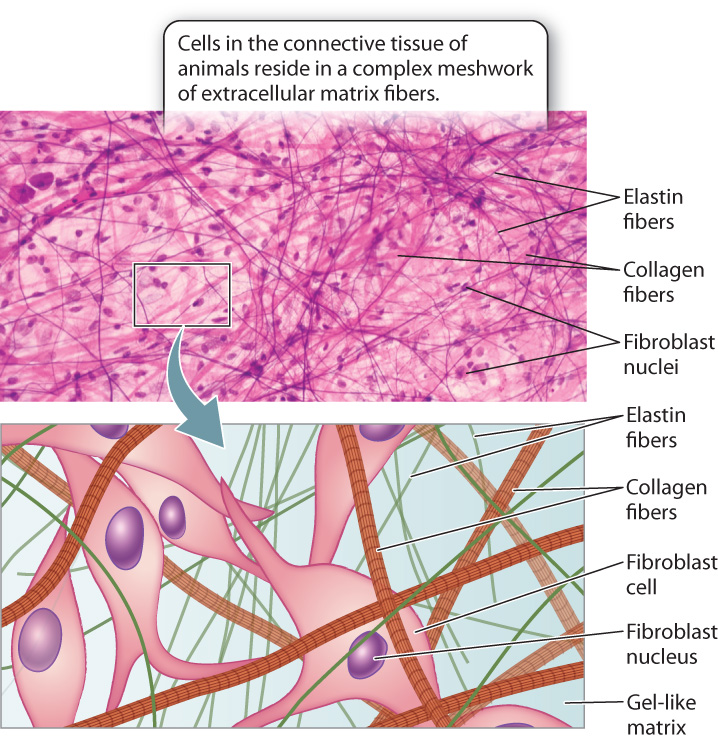
The extracellular matrix can be found in abundance in animal connective tissue (Fig. 10.19). Connective tissue underlies all epithelial tissues, as we have seen. For example, the dermis of the skin is connective tissue, providing support and nutrients to the overlying epidermis. A number of cell types, including the fibroblasts that synthesize most of the extracellular matrix proteins, reside in this tissue. Connective tissue is unusual compared to other tissue types in that it is dominated by the extracellular matrix and has a low cell density. Consequently, the extracellular matrix determines the properties of different types of connective tissue. Other more specialized types of animal connective tissue include bone, cartilage, and tendon.
Collagen is the most abundant protein in the extracellular matrix of animals, and in fact is the most abundant animal protein on the planet. There are more than 20 different forms of collagen, and in humans collagen accounts for almost a quarter of the protein present in the body. Over 90% of this collagen is type I collagen, which is present in the dermis of your skin, where it provides strong, durable support for the overlying epidermis. The tendons that connect your muscles to bones and the ligaments that connect your bones to other bones are able to withstand the physical stress placed on them because they are made up primarily of collagen.
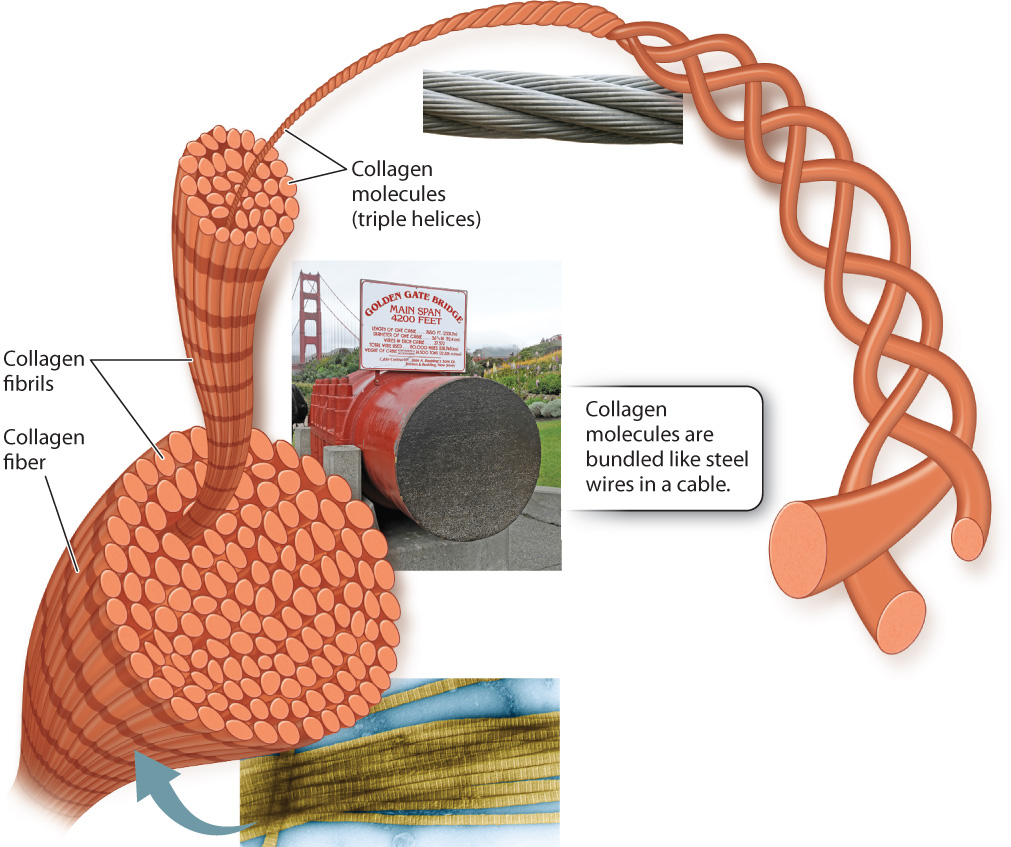
Collagen’s strength is related to its structure. Like a rope or a cable, collagen is composed of intertwined fibers that make it much stronger than if it were a single fiber of the same diameter. A collagen molecule consists of three polypeptides wound around one another in a triple helix. A bundle of collagen molecules forms a fibril, and the fibrils are assembled into fibers (Fig. 10.20). Once multiple collagen fibers are assembled into a ligament or tendon, the final structure is incredibly strong.
10.5.3 The basal lamina is a special form of extracellular matrix.
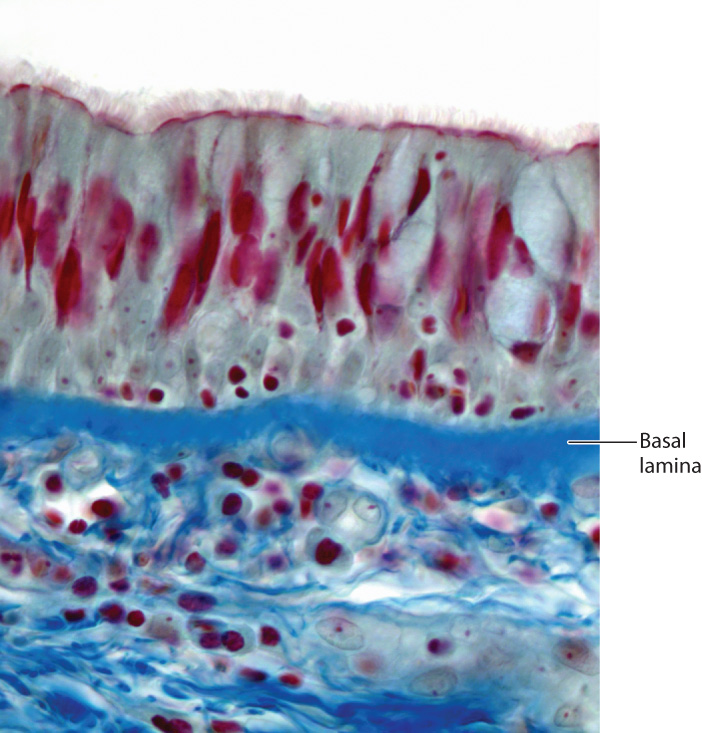
The basal lamina is a specialized layer of extracellular matrix that is present beneath all epithelial tissues, including the lining of the digestive tract, epidermis of the skin, and endothelial cells that line the blood vessels of vertebrates (Fig. 10.21). The role of the basal lamina is to provide structural foundation for these epithelial tissues. The basal lamina is made of several proteins, including a special type of collagen. The triple-helical structure of collagen provides flexible support to the epithelial sheet and also provides a scaffold on which other proteins are assembled.
Epithelial cells are firmly anchored to the basal lamina by a version of the desmosome called a hemidesmosome (see Fig. 10.17). Integrins are the prominent cell adhesion molecules in hemidesomsomes. Their extracellular domains bind to the extracellular matrix proteins in the basal lamina, and their cytoplasmic domains are linked to intermediate filaments of the cytoskeleton. The intermediate filaments connected to hemidesomsomes are linked to those connected to the desmosomes in the lateral membrane. The result is a firmly anchored and tremendously reinforced layer of cells.
A migrating cell must be able to attach to extracellular matrix proteins in order to move forward. These contacts are also made by means of integrins. Cell migration in animals is normal and necessary during embryonic development (Chapter 42), wound healing, and the immune response to infection (Chapter 43). However, inappropriate cell migration can have devastating consequences, as we discuss now.
Case 2 Cancer: When Good Cells Go Bad
10.5.4 How do cancer cells spread throughout the body?
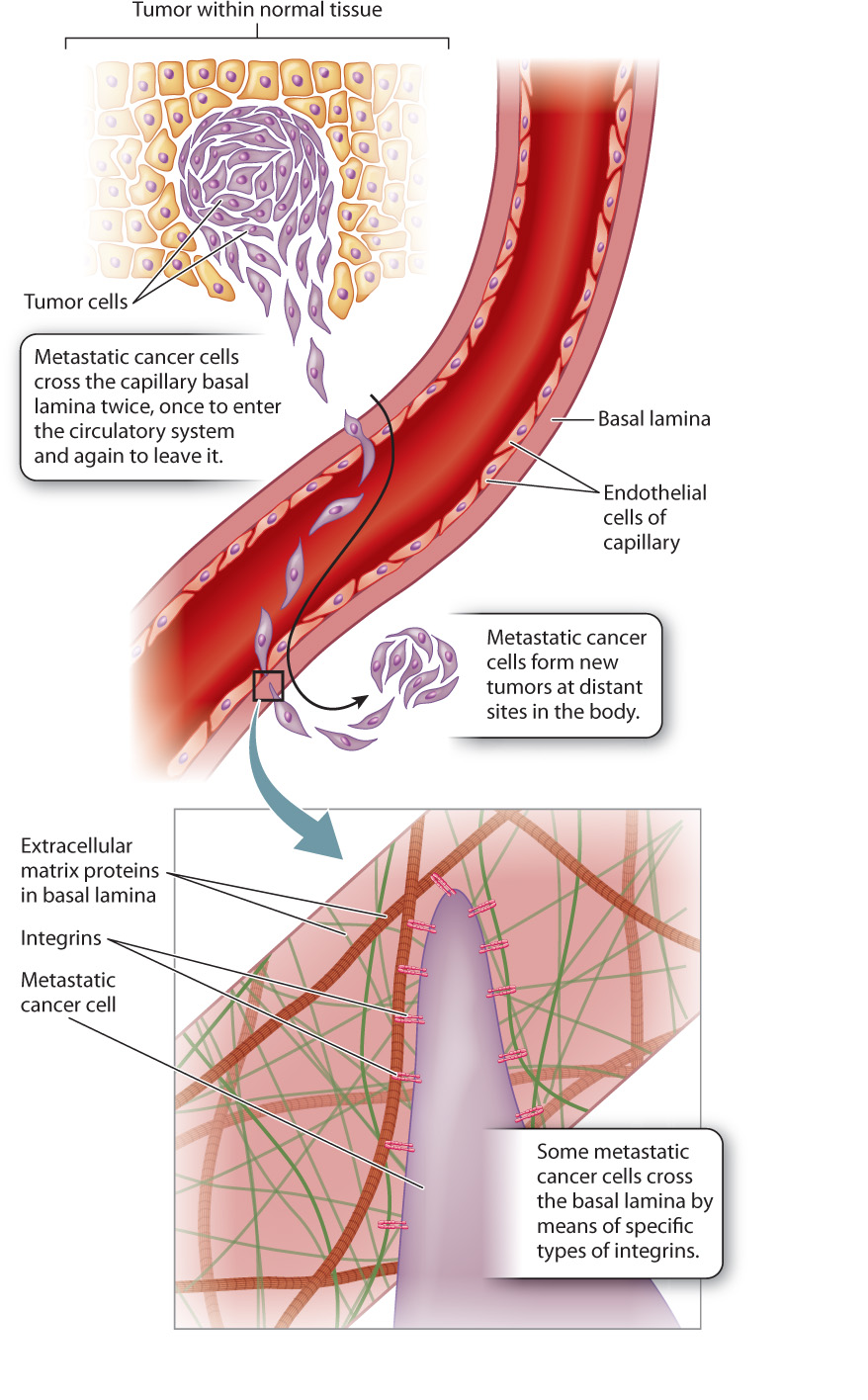
Nonmalignant, or benign, tumors are encapsulated masses of cells that divide continuously because regulation of cell division has gone awry (Chapter 11). As the tumor grows, its border pushes outward against adjacent tissues. Benign tumors are rarely life threatening unless the tumor interferes with the function of a vital organ.
Malignant tumors are different. They contain some cells that can metastasize, that is, break away from the main tumor and colonize distant sites in the body. Metastatic tumor cells have an enhanced ability to adhere to extracellular matrix proteins, especially those in the basal lamina. This is significant because for a cell to metastasize, it must enter and leave the bloodstream through capillaries. Since all blood vessels, including capillaries, have a basal lamina, a metastatic tumor cell needs to cross a basal lamina at least twice—once on the way into the bloodstream and again on the way out (Fig. 10.22). Since cells attach to basal lamina proteins by means of integrins, many studies have compared the integrins in metastatic and non-metastatic cells in the search for potential targets for treatment.
In some types of cancer, the number of specific integrins on the cell surface is an indicator of metastatic potential. Melanoma provides an example. A specific type of integrin is present in high amounts on metastatic melanoma cells but is absent on non-metastatic cells from the same tumor. In laboratory tests, blocking these integrins eliminates the melanoma cell’s ability to cross an artificial basal lamina. Drugs targeting this integrin protein are currently in clinical trials.
10.5.5 Extracellular matrix proteins influence cell shape and gene expression.
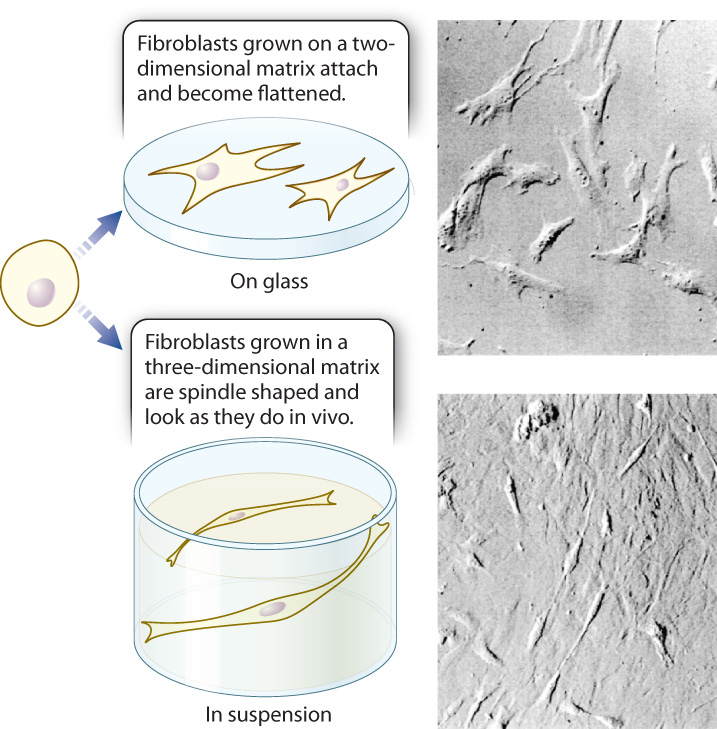
The extracellular matrix is not an inert, three-dimensional environment in which cells live; instead, it plays an active role. Cells continue to interact with the extracellular matrix long after they have synthesized it or moved into it, and these interactions can have profound effects on the cell. Biologists have observed the results of these interactions in experiments conducted with cells grown in culture in the laboratory. For example, fibroblasts that secrete components of the extracellular matrix are in turn influenced by the extracellular matrix. Fibroblasts cultured on a two-dimensional surface coated with extracellular matrix proteins attach to the matrix and flatten as they maximize their adhesion to the matrix. By contrast, the same cells cultured in a three-dimensional gel of extracellular matrix look and behave like the spindle-shaped, highly migratory fibroblasts present in living connective tissue (Fig. 10.23). So the structure of the extracellular matrix can influence the shape of cells.
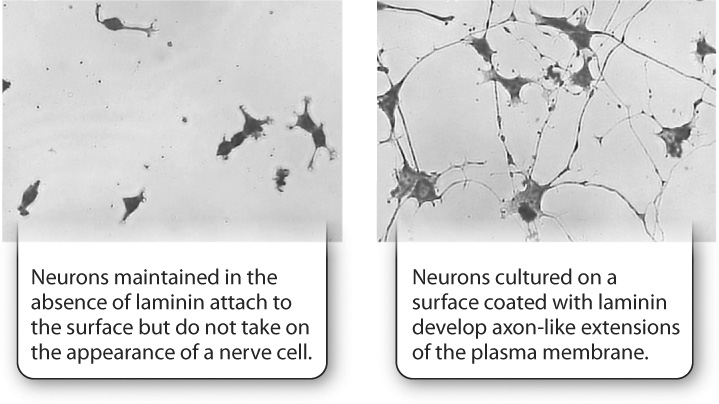
A second example is provided by nerve cells. When these cells are grown in culture on a plastic surface, they attach to the surface of the dish, but do not take on a neuron-like shape. However, when these cells are grown on the same surface coated with the extracellular matrix protein laminin, they develop long extensions that resemble the axons and dendrites of normal nerve cells (Fig. 10.24). In this case, the composition of the extracellular matrix can affect the shape of cells.
In addition to influencing cell shape, the structure and composition of the extracellular matrix can influence gene expression of the cells that are grown in it. Mouse mammary epithelial cells grown in culture on plain glass coated with collagen remain alive and apparently healthy, and even make and secrete a minimal amount of milk proteins, including β-casein, when stimulated by the milk-inducing hormone prolactin. However, if the mammary epithelial cells are grown in three-dimensional collagen gels, they synthesize and secrete up to 10 times more β-casein.
Quick Check 4
Do you think cadherins or integrins are responsible for the dependence of mammary cells on the extracellular matrix for their ability to produce milk proteins? Why?
Two related experiments that further explore the importance of extracellular matrix proteins in the regulation of gene expression are described in Fig. 10.25. Taken together, these experiments demonstrate that there is a dynamic interplay between the extracellular matrix and the cells that synthesize it.
FIG. 10.25Can extracellular matrix proteins influence gene expression?
BACKGROUND The adhesion of cells to the extracellular matrix is required for cell division, DNA synthesis, and proper cell shape. Research by Iranian-American cell biologist Mina Bissell and colleagues indicated that cellular interaction with extracellular matrix proteins influences gene expression. Bissell discovered that mammary cells expressed and secreted high levels of the milk protein β-casein when grown in a three-dimensional collagen matrix but not in a two-dimensional collagen matrix. American cell biologist Joan Caron followed up these studies using liver cells called hepatocytes.
HYPOTHESIS Caron hypothesized that a specific protein in the extracellular matrix is necessary for the expression of the protein albumin from hepatocytes grown in culture. Albumin is a major product of liver cells.
EXPERIMENT Caron cultured hepatocytes on a thin layer of type I collagen, which does not induce albumin synthesis. Next, she added a mixture of several different extracellular matrix proteins to the culture and looked for changes in albumin gene expression and protein secretion into the media. She then tested individual extracellular matrix proteins from the mixture to see which one was responsible for the increase in albumin gene expression.
RESULTS Caron found that when she cultured cells on collagen with a combination of three extracellular matrix proteins—laminin, type IV collagen, and heparin sulfate proteoglycan (HSPG)—the cells synthesized albumin mRNA and secreted albumin protein for several weeks, but if she cultured the cells on collagen alone, they did not (top and middle graphs). In addition, when she tested individual extracellular matrix proteins, she found that laminin, but not any of the other proteins, caused an increase in albumin gene expression (bottom graph).
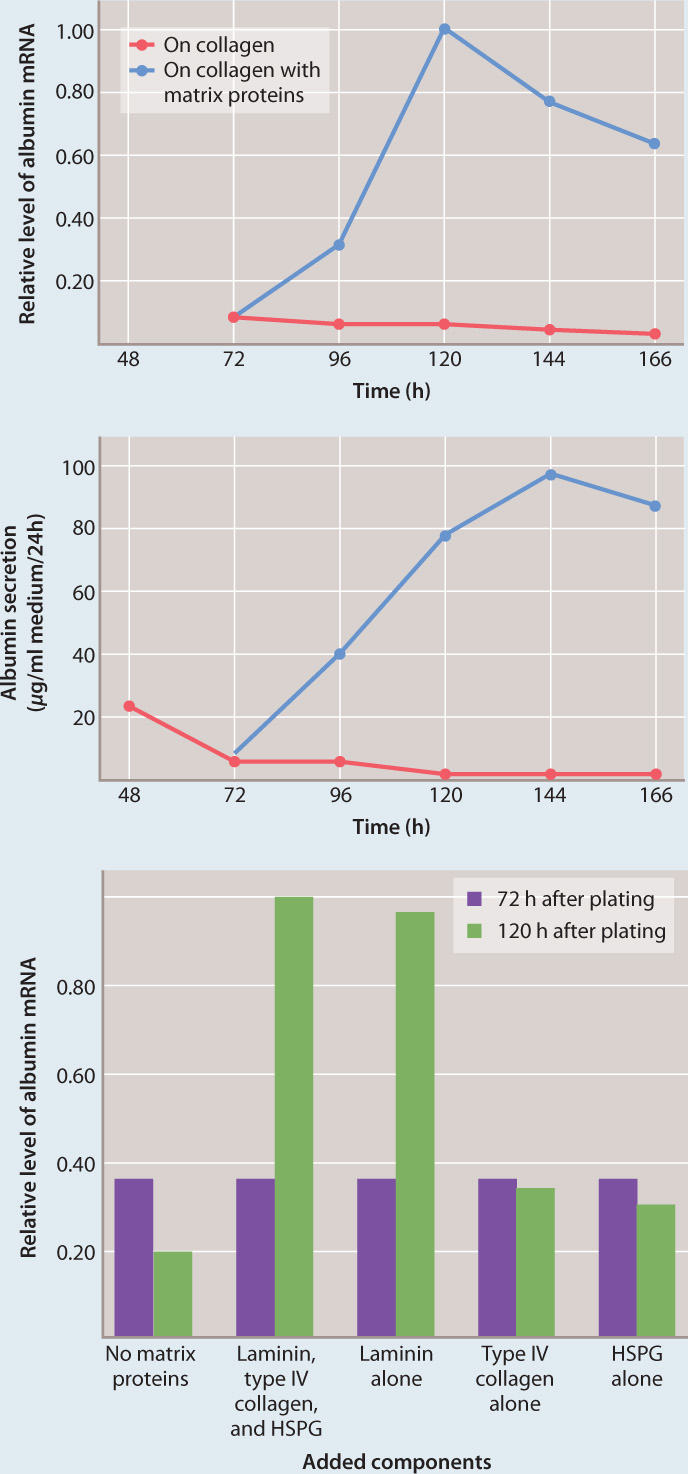
CONCLUSION Caron’s hypothesis was supported by the experiments. A specific extracellular matrix protein, laminin, influences the expression of albumin by hepatocytes.
FOLLOW-UP WORK Bissell continued her work with mammary cells and found that the expression of the β-casein gene was also increased by laminin in the same way as the albumin gene in hepatocytes.
SOURCES Lee, E. Y., W. H. Lee, C. S. Kaetzel, G. Parry, and M. J. Bissell. 1985. “Interaction of Mouse Mammary Epithelial Cells with Collagen Substrata: Regulation of Casein Gene Expression and Secretion.” PNAS 82 : 1419–1423; Caron, J. M. 1990. “Induction of Albumin Gene Transcription in Hepatocytes by Extracellular Matrix Proteins.” Molecular and Cellular Biology 10 : 1239–1243.