14.1 THE RATE AND NATURE OF MUTATIONS
Most mutations are spontaneous, occurring by chance in the absence of any assignable cause. They also occur randomly, unconnected to an organism’s needs—it makes no difference whether or not a given mutation would benefit the organism. Whether a favorable mutation does or does not occur is purely a matter of chance. This key principle, that mutations are spontaneous and random, as well as other basic features of mutation, are discussed in this section.
14.1.1 For individual nucleotides, mutation is a rare event.
For any individual nucleotide, mutation is a very rare event, and the rate of mutation varies among organisms. Fig. 14.1 compares the probability of the occurrence of a new mutation in a given base pair in a single round of replication in different organisms. As seen in the graph, the mutation rates for different organisms range across almost eight orders of magnitude.
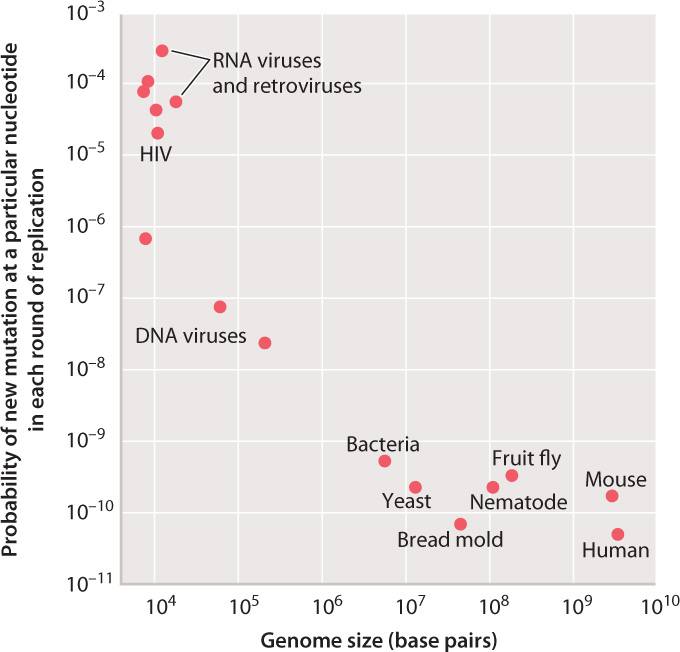
The highest rates of mutation per nucleotide per replication are found among RNA viruses and retroviruses, including HIV. Lower rates occur in DNA viruses, and even lower rates in unicellular organisms such as bacteria and yeast. The rates of mutation per nucleotide per DNA replication are nearly the same for all multicellular animals, including mice and humans.
It is unclear why the rates vary over such a large range. RNA viruses and retroviruses might be expected to have a relatively high rate of mutation, first because the backbone of an RNA strand is more prone to spontaneous breakage than that of a DNA strand, and second because the replication of these genomes lacks a proofreading function. For the other genomes, the rate of mutation per nucleotide per DNA replication might simply reflect the limits of proofreading and other repair mechanisms. Some mutations must inevitably occur because of chemical errors such as base mispairing and DNA-strand breakage.
For the cellular organisms plotted in Fig. 14.1, the average probability of a new mutation per DNA replication is about 10–10, which means that, on average, only 1 nucleotide in every 10 billion is mistakenly substituted for another.
But averaging conceals many details. First, certain nucleotides are especially prone to mutation and can exhibit rates of mutation that are greater than the average by a factor of 10 or more. Sites in the genome that are especially mutable are called hotspots. Second, in some multicellular organisms, the rates of mutation differ between the sexes. In humans, for example, the rate of mutation is substantially greater in males than in females. Finally, the rates for the multicellular animals plotted in Fig. 14.1 depend on the type of cell: A distinction must be made between mutations that occur in germ cells (the reproductive cells and the cells that give rise to them) and mutations that occur in somatic cells (the other cells of the body). In mammals, the rate of mutation per nucleotide per replication is greater in somatic cells than in germ-line cells.
14.1.2 Across the genome as a whole, mutation is common.
So far, we have considered the mutation rate for any given nucleotide, which varies from organism to organism, but in all cases is relatively small. For a particular cell, the overall mutation rate depends on the number of times DNA replication occurs and the size of the genome. In multicellular organisms, there are sometimes many cycles of DNA replication and cell division that take place in the germ line before meiosis and the formation of reproductive cells. In a human male at age 30, for example, about 400 cycles of DNA replication and cell division take place before meiosis. Therefore, the overall rate of mutation per nucleotide in sperm is 400 times greater than the rate per single cycle of replication plotted in Fig. 14.1. Furthermore, some genomes are much larger than others (Chapter 13), and across a genome as a whole, there will be a greater number of mutations per genome in large genomes than in small ones.
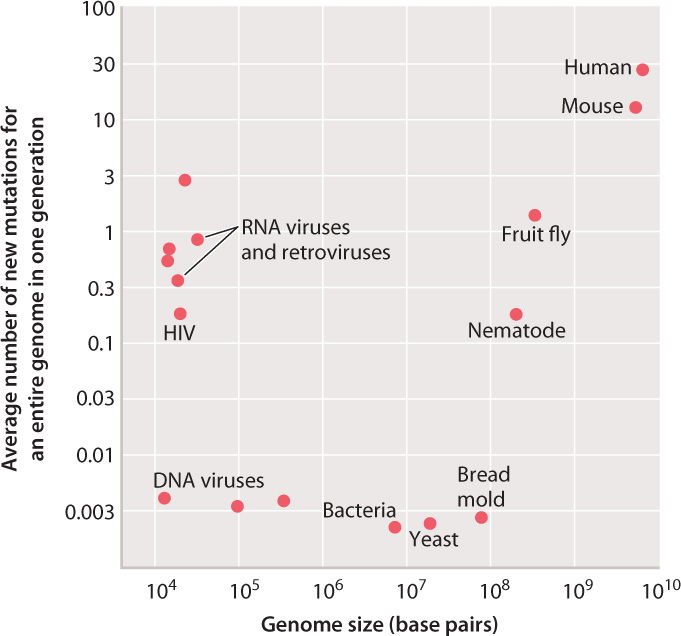
Fig. 14.2 shows the average number of mutations for a genome in an organismal generation for the same organisms as shown in Fig. 14.1, but in this case taking into account the number of germ-line cell divisions as well as genome size. The picture is now quite different. For the DNA viruses, bacteria, and fungi (the bread mold), the average number of new mutations per genome per generation is remarkably similar, about 0.003. This number indicates that, in bacteria and fungi, roughly one cell division in every eight yields a daughter cell with a new mutation somewhere in the genome.
For RNA viruses and retroviruses, the average number of new mutations per genome reflects the high rate of mutation per nucleotide seen in Fig. 14.1. Among multicellular animals, the average number of new mutations per genome reflects the effects of multiple germ-line cell divisions per generation of organisms as well as larger genome size. It is these mutations, accumulated through many generations and shuffled by recombination and independent assortment (Chapter 11), that account for the genetic diversity of most populations and the genetic uniqueness of each individual.
Such a large number of new mutations as occurs in the human genome would be intolerable in organisms with a high density of protein-coding genes, such as bacteria or fungi. It is tolerable in humans and other mammals only because a mere 2.5% of the genome codes for protein (Chapter 13). The vast majority of new mutations therefore occur in noncoding DNA and are neutral or very nearly neutral in their effects.
14.1.3 Only germ-line mutations are transmitted to progeny.
Which is more important—the rate of mutation per cycle of DNA replication or the rate of mutation per organismal generation? That depends on context. Mutations can take place in any type of cell. Those that occur in eggs and sperm and the cells that give rise to these reproductive cells are called germ-line mutations, and those in nonreproductive cells are called somatic mutations. This distinction is important because somatic mutations affect only the individual in which they occur. In other words, they are not transmitted to future generations. In contrast, germ-line mutations are transmitted to future generations because they occur in reproductive cells.
For germ-line mutations, it is the rate of mutation per organismal generation that matters most. Germ-line mutations are important to the evolutionary process because, through transmission between generations, they may eventually come to be present in many individuals descended from the original carrier.

For somatic mutations, the mutation rate that matters is the rate of mutation per cycle of DNA replication. Although somatic mutations are not transmitted to future generations, they are transmitted to progeny cells in mitotic cell divisions (Chapter 11). Hence, a somatic mutation affects not only the cell in which it occurs, but also all the cells that descend from it. The areas of different color or pattern that appear in “sectored” flowers, valued as ornamental plants (Fig. 14.3), are usually due to somatic mutations in flower-color genes. A mutation in a flower-color gene occurs in one cell, and as it replicates during development of the flower, all its descendants in the cell lineage—the generations of cells that originate from a single ancestral cell—carry that mutation, producing a sector with altered coloration.
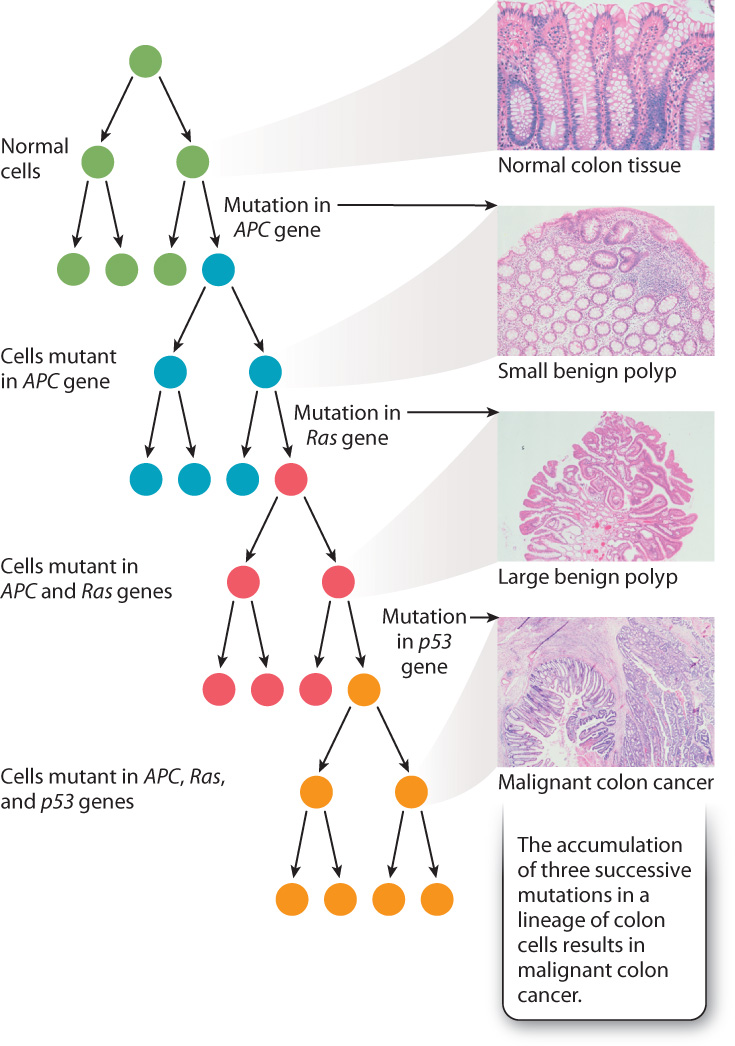
Most cancers result from mutations in somatic cells (Chapter 11). In some cases, the mutation increases the activity of a gene that promotes cell growth and division, while in other cases, it decreases the activity of a gene that restrains cell growth and division. In either event, the mutant cell and its descendants escape from one of the normal control processes. Fortunately, a single somatic mutation is usually not sufficient to cause cancer—usually two or three or more mutations in different genes are required to derail control of normal cell division so extensively that cancer results.
To cause cancer, the mutations must all be present in the same cell lineage, which means that they all must have occurred in the same original cell. Fig. 14.4 shows three key mutations that have been implicated in the origin of invasive colon cancer: p53, Ras, and APC. Each mutation occurs randomly, but if, by chance, a mutation in the Ras gene occurs in a cell that is derived from one in which the APC gene has been mutated, that cell’s progeny forms a polyp. Another chance mutation in the same cell line, which now carries mutations in both the APC and Ras genes, could lead to malignant cancer. Normally, the occurrence of multiple mutations in a single cell lineage is rare, but in people exposed to chemicals that cause mutations or who carry mutations in DNA repair processes, multiple mutations in a single cell lineage are more likely to occur and so the risk of cancer is increased.
Case 3 You, From A To T: Your Personal Genome
14.1.4 What can your personal genome tell you about your genetic risk factors?
Cancer is usually due to a series of mutations that occur sequentially in a single lineage of somatic cells, as illustrated for colon cancer in Fig. 14.4. Each type of cancer is caused by its own particular sequence of mutations, although some genes are implicated in several different types of cancer. An example is the p53 gene, the product of which detects DNA damage and slows the cell cycle to allow time for DNA repair (Chapter 11). Mutations in p53 are one step in the mutational progression of many different types of cancer, including colon cancer and breast cancer.
In most individuals with cancer, all the sequential mutations that cause the cancer are spontaneous mutations that take place in somatic cells. They are not transmitted through the germ line, and so there is little or no increased risk of cancer in the offspring. In some families, however, there is a germ-line mutation in one of the genes implicated in cancer that is transmitted from parents to their children. In any child who inherits the mutation, all cells in the body contain the defective gene, and hence the cells already have taken one of the mutational steps that lead to cancer. The effect of such a germ-line mutation is therefore to reduce the number of additional mutations that would otherwise be necessary to produce cancer cells.
Any mutation that increases the risk of disease in an individual is known as a genetic risk factor for that disease. For colon cancer, the major genetic risk factors are mutations in APC, Ras, and p53. For breast cancer, the major genetic risk factors are mutations in the BRCA1 and BRCA2 genes (Case 3: You, From A to T). A risk factor does not cause the disease, but it makes the disease more likely to occur. For the genes implicated in colon cancer, each is a risk factor because, when a mutation is present, only two more mutations are needed for the cancer to form, whereas in its absence three mutations are required. Because a cell lineage is much more likely to undergo two mutations than three, a mutation in each of the genes implicated in colon cancer is a risk factor that substantially increases the likelihood of the disease.
The DNA sequence of each of our personal genomes can reveal the genetic risk factors that each of us carries, not only for cancer but for many other diseases as well. Not all genetic risk factors are known for all diseases, and a great deal of current research aims to identify new ones. But many genetic risk factors are already known for a large number of common diseases, including high blood pressure, diabetes, inflammatory bowel disease, age-related macular degeneration, Alzheimer’s disease, and many forms of cancer. Therefore, your personal genome can be of great value in identifying diseases for which you carry risk factors, as was the case for Claudia Gilmore (Case 3: You, From A to T).
Our personal genomes can identify only genetic risk factors, however. In many cases, disease risk is substantially increased by environmental risk factors as well, especially lifestyle choices. While there are genetic risk factors for lung cancer, for example, the single biggest environmental risk factor is cigarette smoking. For skin cancer, the greatest environmental risk factor is exposure to the damaging ultraviolet rays in sunlight. For heart disease, it is smoking, lack of physical activity, and obesity. For diabetes, it is an unhealthy diet.
For breast cancer, the environmental risk factors include certain forms of hormone therapy, lack of physical activity, and alcohol. While we may not be able to do much about the genetic risk factors for any of these conditions, knowing that we have them may make us more careful about the lifestyle choices that we make.
14.1.5 Mutations are random with regard to an organism’s needs.
How do mutations arise? Consider the following example: If an antibiotic is added to a liquid culture of bacterial cells that are growing and dividing, most of the cells are killed, but a few survivors continue to grow and divide. These survivors are found to contain mutations that confer resistance to the antibiotic. This simple observation raises a profound question. Does this experiment reveal the presence of individual bacteria with mutations that had arisen spontaneously and were already present? Or do the antibiotic-resistant mutants arise in response to the presence of the antibiotic?
These alternative hypotheses had deep implications for all of biology because they suggested two very different ways in which mutations might arise. The first suggested that mutations occur without regard to the needs of an organism. According to this hypothesis, the presence of the antibiotic in the experiment with bacterial cells would not direct or induce antibiotic resistance in the cells, but instead would allow the small number of preexisting antibiotic-resistant mutants to flourish at the expense of the antibiotic-sensitive cells. The second hypothesis suggested that there is some sort of feedback between the needs of an organism and the process of mutation, and the environment directs specific mutations that would be beneficial to the organism.
To distinguish between these two hypotheses, in 1952 Joshua and Esther Lederberg carried out a now-famous experiment, described in Fig. 14.5. Bacterial cells were grown and formed colonies on agar plates in the absence of antibiotic. Then, using a technique they invented called replica plating, the Lederbergs transferred these colonies to new plates containing antibiotic. Only bacteria that were resistant to the antibiotic grew on the new plates. Because replica plating preserved the arrangement of the colonies, the Lederbergs were able to go back to the original plate and identify the colony that produced the antibiotic-resistant colony on the replica plate. From that original colony, they were then able to isolate a pure culture of antibiotic-resistant bacteria.
FIG. 14.5Do mutations occur randomly, or are they directed by the environment?
BACKGROUND Researchers have long observed that beneficial mutations tend to persist in environments where they are useful—in the presence of antibiotic, bacteria become antibiotic resistant; in the presence of insecticides, insects become insecticide resistant.
HYPOTHESES These observations lead to two hypotheses about how a mutation, such as one that confers antibiotic resistance on bacteria, might arise. The first suggests that mutations occur randomly in bacterial populations and over time become more common in the population in the presence of antibiotic (which destroys those bacteria without the mutation). In other words, they occur randomly with respect to the needs of an organism. The second hypothesis suggests that the environment, in this case the application of antibiotic, induces or directs antibiotic resistance.
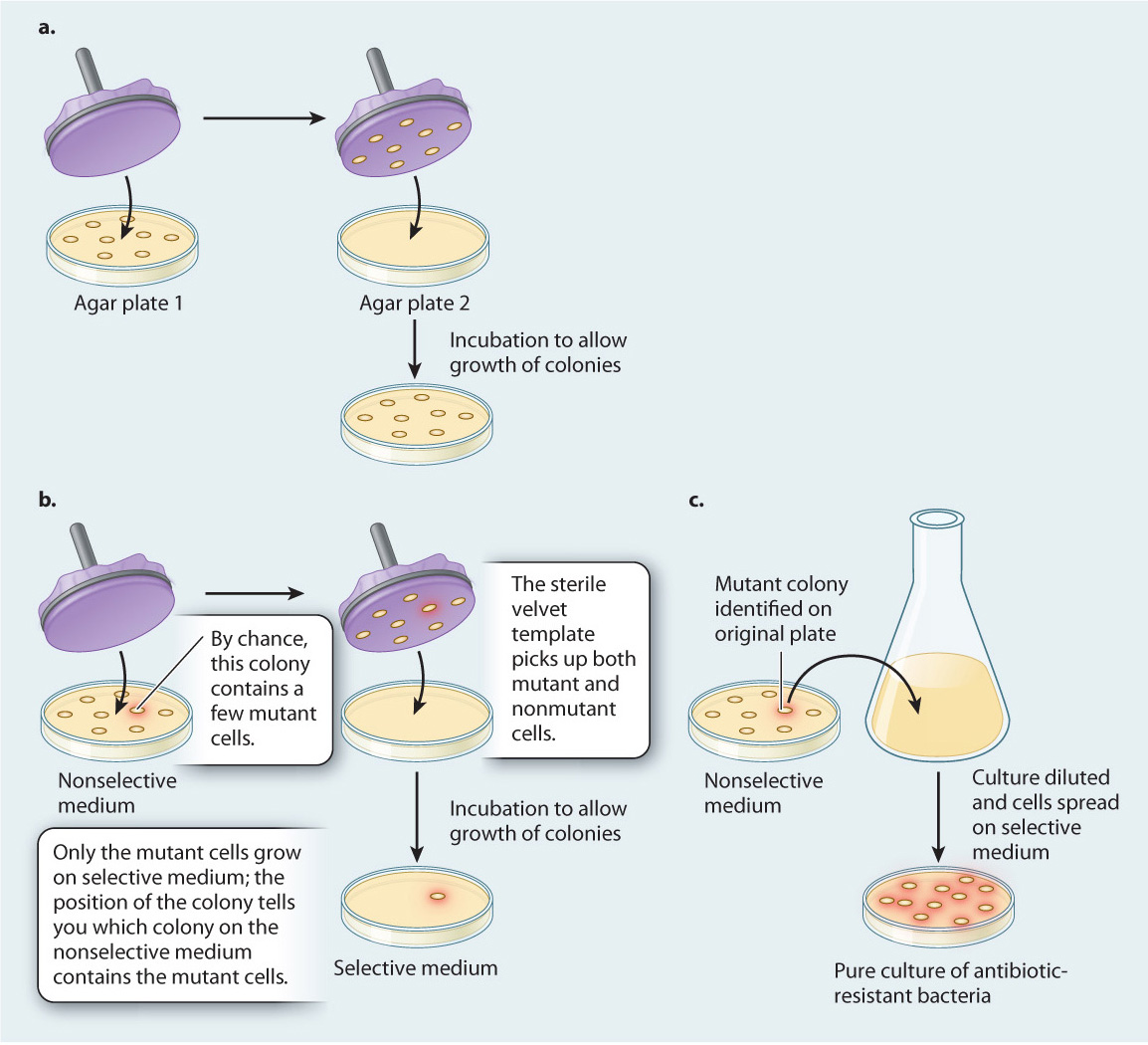
METHOD To distinguish between these two hypotheses, Joshua and Esther Lederberg developed replica plating. In this technique, bacteria are grown on agar plates, where they form colonies (Fig. 14.5a). The cells in any one colony result from the division of a single original cell, and thus they constitute a group of cells that are genetically identical except for rare mutations that occur in the course of growth and division. Then a disk of sterilized velvet is pressed onto the plate. Cells from each colony stick to the velvet disk (in mirror image, but the relative positions of the colonies are preserved). The disk is then pressed onto the surface of a fresh plate, transferring to the new plate a few cells that originate from each colony on the first agar plate, in their initial positions.
EXPERIMENT First, the Lederbergs grew bacterial colonies on medium without antibiotic, called a nonselective medium because all cells are able to grow and form colonies on it. Then, by replica plating, they transferred some cells from each colony to a plate containing antibiotic, so only antibiotic-resistant cells could multiply and form colonies. (Medium containing antibiotic is a selective medium because it “selects” for a particular attribute or element, in this case antibiotic-resistant cells.) Because replica plating preserves the arrangement of the colonies, the location of an antibiotic-resistant colony on the selective medium reveals the location of its parental colony on the nonselective plate (Fig. 14.5b). Finally, the Lederbergs were able to go back to the original colony and isolate a pure culture of antibiotic-resistant bacteria (Fig. 14.5c).
CONCLUSION The Lederbergs’ replica-plating experiments demonstrated that antibiotic-resistant mutants can arise in the absence of antibiotic because at no time in the experiments did the cells on nonselective medium come into contact with the antibiotic. Only the successive generations of daughter cells carried over to selective medium by replica plating were exposed to the antibiotic. Nevertheless, by their procedure the Lederbergs were able to isolate pure colonies of antibiotic-resistant cells.
FOLLOW-UP WORK These results have been extended to other types of mutation and other organisms, suggesting that mutations are random and not directed by the environment.
SOURCE Lederberg, J., and E. M. Lederberg. 1952. “Replica Plating and Indirect Selection of Bacterial Mutants.” Journal of Bacteriology 63:399–406 .
Replica plating allowed the Lederbergs to isolate a pure culture of antibiotic-resistant bacteria, even though the original bacteria actually never were exposed to antibiotic. This result supported the first hypothesis: Mutations occur randomly, and without regard to the needs of the organisms. The role of the environment is not to create specific mutations, but instead to select them. The principle the Lederbergs demonstrated is true of all organisms so far examined.
Quick Check 1
If mutations occur at random with respect to an organism’s needs, then how does a species become more adapted to its environment over time?