18.4 COMPLEX TRAITS IN HEALTH AND DISEASE
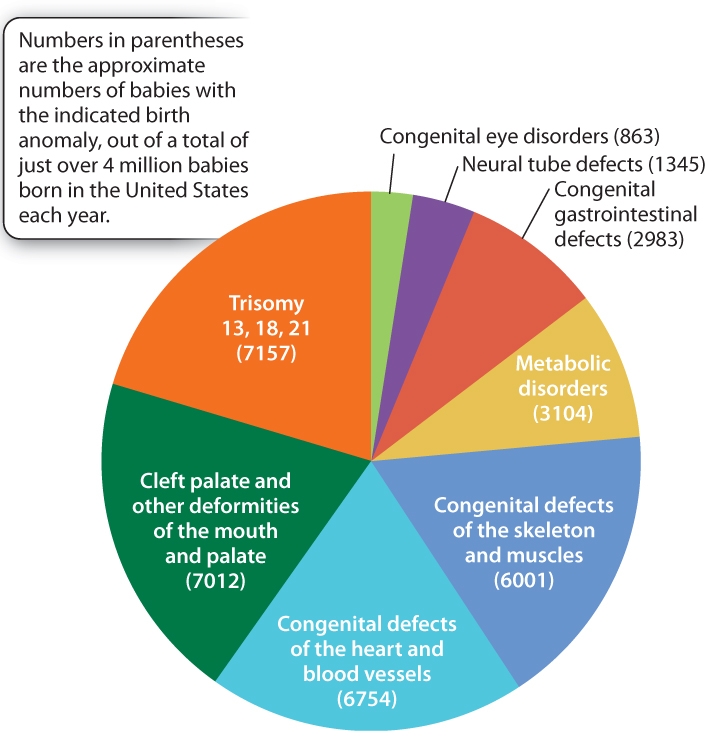
Much evidence beyond twin studies implies an important role for particular alleles as risk factors for diabetes, high blood pressure, asthma, rheumatoid arthritis, epilepsy, schizophrenia, clinical depression, autism, and many other conditions. These are all complex traits, affected by genotype, environment, and genotype-by-environment interactions. Even the most common birth defects are affected by multiple genetic risk factors. The most common birth anomalies and the numbers of affected babies born in the United States each year—collectively more than 35,000—are indicated in Fig. 18.12. About 20% of these anomalies are due to an extra chromosome, and trisomy 21 (Down syndrome) is by far the most common. Another roughly 10% are due to defects in metabolism. Many of these—among them diseases such as cystic fibrosis, sickle-cell anemia, and phenylketonuria (the inability to break down the amino acid phenylalanine)—are rare simple Mendelian disorders. But the majority of birth anomalies are affected by both genetic and environmental risk factors.
18.4.1 Most common diseases and birth defects are affected by many genes that each have relatively small effects.
Because the most common birth anomalies as well as childhood and adult disorders are complex traits, biologists are keenly interested in identifying the genes that contribute to differences in risk, understanding what these genes do, and translating this knowledge into prevention or treatment. They have therefore begun to apply modern molecular methods—genome sequencing (Chapter 13), genome annotation (Chapter 13), and genotyping (Chapter 15)—to complex traits.
The identification of genes affecting complex traits is not only a major goal of much current research in human genetics, but also in domesticated animals and plants. Gene identification is also important in model organisms used in research, especially the laboratory mouse. Much is already known about these model organisms and they are easily manipulated, making it possible to discover the molecular mechanisms by which genes affecting complex traits exert their effects.
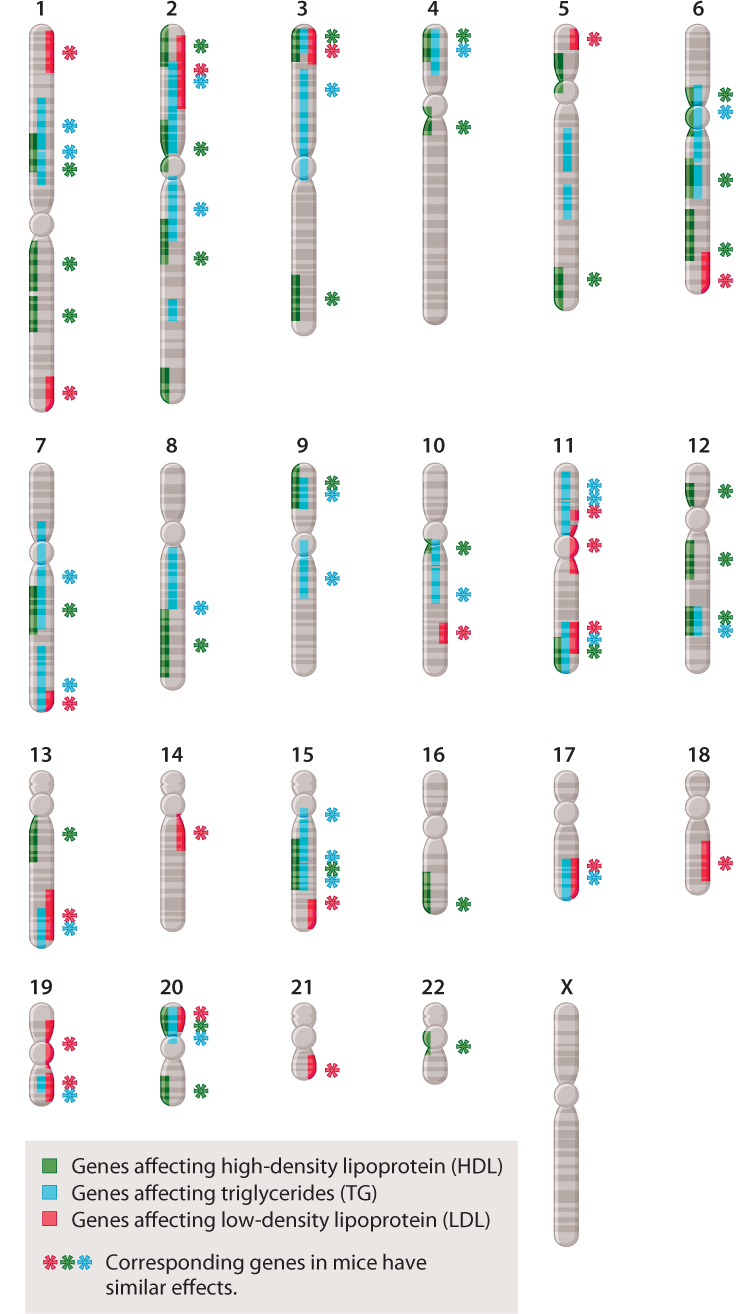
The study of complex traits has already reached a stage at which patterns are beginning to emerge. Many of these patterns are exemplified by the chromosome map in Fig. 18.13, which shows the location of genes in the human genome that affect cholesterol levels. The first observation is that many genes contribute to cholesterol levels in humans—specifically, more than 50 genes contribute to the level of HDL (high-density lipoproteins, or “good cholesterol”), LDL (low-density lipoproteins, or “bad cholesterol”), or triglycerides. A second observation is that many of these genes affect two or even all three of the types of molecule. (A single gene that has multiple effects is said to show pleiotropy.) Third, many of the genes show epistasis, that is, they interact with one another (Chapter 16). Fourth, many of the genes occur in clusters, being physically close together in the same chromosome. Clustering of genes reflects the fact that many genes arose through the process of duplication of a single gene followed by divergence over time, generating a family of genes near one another with related functions (Chapter 14). Finally, the functions of all of the genes that contribute to a complex trait are not known. Some of the genes in Fig. 18.13 affect serum cholesterol through known metabolic pathways, but many others work through unknown pathways. Many of the human genes shown have counterparts in the mouse genome that have similar effects on serum cholesterol, so these genes are open to direct experimental investigation.
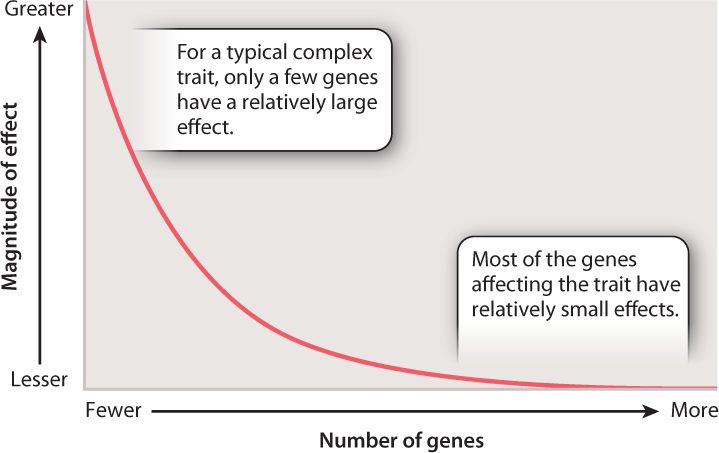
A principle that is not evident in Fig. 18.13 is that the effects of the genes on cholesterol levels are very unequal. Some have relatively large effects, while others have small ones. The distribution of the magnitude of gene effects for complex traits resembles that shown in Fig. 18.14. The axes are labeled only in relative terms, because the actual numbers and effect sizes differ from one trait to the next. However, the main point is that for the majority of genes that contribute to a complex trait, the magnitude of their individual effects is typically quite small. The magnitude of genetic effects also often differs between the sexes, which helps explain why complex traits so often differ in prevalence or severity between males and females.
Quick Check 3
When genes for complex traits have effects that are distributed as shown in Fig. 18.14, which are easier to identify: those that are numerous with small effects, or those that are few with large effects?
18.4.2 Human height is affected by hundreds of genes.
For most complex traits, the genes that have been identified to date account for only a relatively small fraction of the total variation in the trait. One extreme example is adult height. An enormous amount of data is available for height, not because height has been studied extensively for its own sake, but because in studies of common diseases the height of each individual is recorded, and hence data on height are available without additional effort or expense.
In one analysis, results from 46 separate studies were combined. These studies included about 185,000 individuals who were genotyped for common nucleotide variants (SNPs) at about 3 million nucleotide sites. The analysis identified at least 180 genes affecting height. Some of these genes are known to affect skeletal development, growth hormones, or other growth factors, but most have no obvious connection to the biology of growth. Some of the genes had previously been identified by studies of rare mutations that have pronounced effects on skeletal growth, either in human families or in laboratory mice. An unexpected finding was that a few genes affecting height are known to be associated with bone mineral density, obesity, and rheumatoid arthritis. These and many other of the genes identified might affect height indirectly.
The 180 genes for height identified among the 185,000 individuals account for only about 12% of the genetic variation in height. The authors calculate that studies of 500,000 individuals would reveal approximately 520 additional genes with effects as large as or larger than those of the 180 genes identified. They also estimate that these 700 genes in total would still account for only 20% of the genetic variation in height, suggesting that many more genes of still smaller effect contribute to variation in height. And this analysis does not address at all the effects of the environment on human height, nor genotype-by-environment interactions, which likely play an important role as well. Complex traits therefore require sophisticated studies to tease apart all the genetic and environmental factors that play a role.
Case 3 You, From A to T: Your Personal Genome
18.4.3 Can personalized medicine lead to effective treatments of common diseases?
The multiple genetic and environmental factors affecting complex traits imply that different people can have the same disease for different reasons. For instance, one person might develop breast cancer because of a mutation in the BRCA1 or BRCA2 genes, while another might develop breast cancer because of other genetic risk factors or even environmental ones. Similarly, one person might develop emphysema because of a mutation in the gene that encodes the enzyme alpha-1 antitrypsin (α1AT) (Chapter 15), and another might have emphysema as a result of cigarette smoking. Other examples where the same disease can be the result of different underlying genetic or environmental factors include elevated cholesterol levels, high blood pressure, and depression. Because the underlying genetic basis for the same disease may be different in different patients, some patients respond well to certain drugs and others do not.
The traditional strategy for treating diseases is to use the same medicine for the same disease. However, because we now know that the same disease may have different causes, another possibility has emerged: to identify each patient’s genotype for each of the relevant genes, and then to match the treatment to the genetic risk factors in each patient. The approach is known as personalized medicine. Personalized medicine matches the treatment to the patient, not the disease.
Personalized medicine not only aims to identify ahead of time medicine that will work effectively, but also to avoid medicines that may lead to harmful side effects, even death. Advertisements and enclosures with prescription drugs enumerate long lists of side effects that you may get if you take a particular medicine. At present, it is difficult to know in advance who will get one or more of these side effects and who won’t—that is, the side effects of taking medicine are themselves complex traits and the result of many underlying genetic and environmental factors. If we could identify ahead of time those patients who will respond negatively to a particular medicine and those who won’t, we could minimize potentially harmful effects of medicines on certain individuals.
Someday, it may be possible to determine each patient’s genome sequence quickly, reliably, and cheaply. At present, personalized medicine is restricted to studies of a few key genes known to have important effects on treatment outcomes. In the treatment of asthma, for example, some of the differences in response to albuterol inhalation have been traced to genetic variation in the gene ADRB2, encoding the β-(beta-)2-adrenergic receptor. Similarly, certain drugs used in the treatment of Alzheimer’s disease are less effective in women with a particular apolipoprotein E (APOE) genotype than in other classes of patients. Also, more than half of the cases of muscle weakness occurring as an adverse effect of drug treatment used to control high cholesterol can be traced to genetic variation in the gene SLCO1B1, which encodes a liver transport protein.
Other factors in addition to genes are involved in these disorders, but genes play an important role. Knowledge of a patient’s genotype can help guide treatment. These are only a few of many examples, but they demonstrate the substantial potential benefits of personalized medicine.