19.3 TRANSCRIPTIONAL REGULATION IN PROKARYOTES
The central message of Fig. 19.1 is that the regulation of gene expression occurs by means of a hierarchy of regulatory mechanisms acting at different levels (and usually at multiple levels) from DNA to protein. Gene regulation in prokaryotes is simpler than gene regulation in eukaryotes since DNA is not packaged into chromosomes, mRNA is not processed, and transcription and translation are not separated by a nuclear envelope. In prokaryotes, expression of a protein-coding gene entails transcription of the gene into messenger RNA and translation of the messenger RNA into protein. Each of these levels of gene expression is subject to regulation.
Because gene regulation is simpler in prokaryotes than gene regulation in eukaryotes, prokaryotes have served as model organisms for understanding how genes are turned on and off. In this section, we consider in more detail how gene expression is regulated at the level of transcription in bacteria and in viruses that infect bacteria. We focus on two well-studied systems: (1) the regulation of genes in the intestinal bacterium Escherichia coli that allows proteins needed to utilize the sugar lactose to be produced only when lactose is present in the environment and only when it is the best nutrient available, and (2) the regulation of genes in a virus that infects E. coli that control whether the virus integrates its DNA into the bacterial host or lyses (breaks open) the cell. In both cases, specific genes are turned on and off in response to environmental conditions.
19.3.1 Transcriptional regulation can be positive or negative.
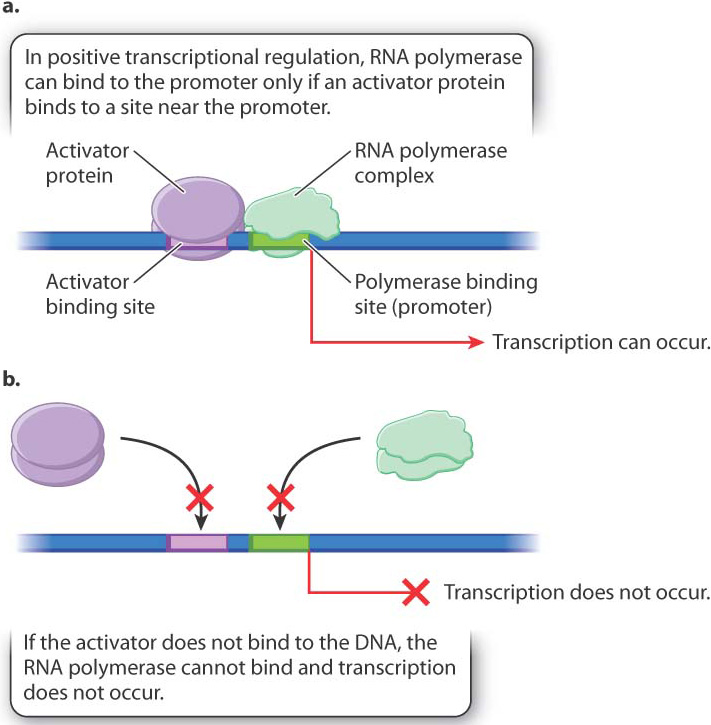
Transcription can be positively or negatively regulated. In positive regulation, a regulatory molecule (usually a protein) must bind to the DNA at a site near the gene in order for transcription to take place. In negative regulation, a regulatory molecule (again, usually a protein) must bind to the DNA at a site near the gene in order for transcription to be prevented.
Fig. 19.12 illustrates positive regulation. The main players are DNA, the RNA polymerase complex, and a regulatory protein called a transcriptional activator. As shown in Fig. 19.12a, when the activator protein is present in a state that can interact with its binding site in the DNA, the RNA polymerase complex is recruited to the promoter of the gene and transcription takes place. When the activator is not present, or not able to bind with the DNA (Fig. 19.12b), transcription does not occur. The binding site for the activator may be upstream of the promoter, as shown in the figure, downstream of the promoter, or even overlap the promoter.
Sometimes, the activator protein combines with a small molecule in the cell and undergoes a change in shape that alters its binding affinity for DNA. The change in shape is an example of an allosteric effect (Chapter 6). In some cases, combining with the small molecule allows the activator to bind with DNA, and the presence of the small molecule in the cell results in transcription of the gene. Genes subject to this type of positive control typically encode proteins needed only when the small molecule is present in the cell. For example, in E. coli, the genes for degradation of the sugar arabinose are regulated by an activator that binds to the sugar. When arabinose is present, the genes are transcribed, but when arabinose is absent, the genes are not transcribed. For some other genes, activator proteins can bind DNA only when a small molecule is absent from the cell. These genes typically encode proteins needed for synthesis of the small molecule. In E. coli, the genes for synthesis of the amino acid cysteine are regulated in this fashion.
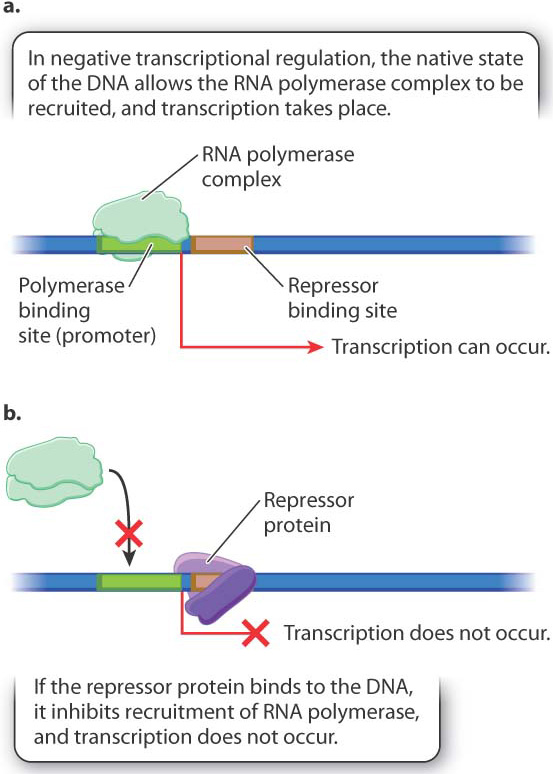
Fig. 19.13 illustrates negative regulation. In this case, the DNA in its native state can recruit the RNA polymerase complex, and transcription takes place at a constant rate unless something turns it off (Fig. 19.13a). What turns it off is binding with a protein called a repressor (Fig. 19.13b). Again, the binding site for the repressor can be upstream from the promoter, downstream, or overlapping.
As with positive control, the active state of the repressor is often determined by an allosteric interaction with a small molecule. A small molecule that prevents binding by the repressor is called an inducer. In the next section, we will look closely at the regulation of genes for the breakdown of the sugar lactose in E. coli, and we will see that lactose binds to and inhibits the repressor and is therefore an inducer of these genes. In other cases, the small molecule changes the conformation of the repressor so that it can bind with the repressor binding site. Genes regulated in this way are often needed for synthesis of the small molecule. In E. coli, for example, the genes for the synthesis of the amino acid tryptophan are negatively regulated by tryptophan. When tryptophan is present in sufficient amounts, it binds with a regulatory protein to form the functional repressor, and transcription of the genes does not occur. When the level of tryptophan drops too low to form the repressor, transcription of the genes is initiated.
Quick Check 3
An activator and inducer both activate gene expression. What’s the difference between an activator and an inducer?
19.3.2 Lactose utilization in E. coli is the pioneering example of transcriptional regulation.
The principle that the product of one gene can regulate transcription of other genes was first discovered in the 1960s by François Jacob and Jacques Monod, who studied how the bacterium E. coli regulates production of the proteins needed for utilization of the sugar lactose. Lactose consists of one molecule each of the sugars glucose and galactose covalently joined by a β-(beta-) galactoside bond. An enzyme called β-galactosidase cleaves lactose, releasing glucose and galactose. Both molecules can then be broken down and used as a source of carbon and energy (Chapter 7).
Jacob and Monod began their research with the observation that active β-galactosidase enzyme is observed in cells only in the presence of lactose or certain molecules chemically similar to lactose. Why is this so? Fig. 19.14 describes and tests two hypotheses. One is that lactose stabilizes an unstable form of β-galactosidase produced by all cells all the time. The other is that lactose leads to the expression of the gene for β-galactosidase. The experiment shown in Fig. 19.14 demonstrated that lactose turns on the β-galactosidase gene and does not stabilize or activate the enzyme encoded by the gene. How lactose activates expression of the β-galactosidase gene was the subject of additional experiments. These Nobel Prize–winning follow-up studies of Jacob and Monod gave the first demonstrated example of transcriptional gene regulation.
FIG. 19.14How does lactose lead to the production of active β-galactosidase enzyme?
BACKGROUND Active β-galactosidase enzyme is observed only in E. coli cells that are growing in the presence of lactose.
HYPOTHESES One hypothesis is that the enzyme is always being produced, but is produced in an unstable form that breaks down rapidly in the absence of lactose. A second hypothesis is that the enzyme is stable, but is produced only in the presence of lactose.
EXPERIMENT François Jacob and Jacques Monod exposed a culture of growing cells to lactose and later removed it. They measured the amount of β-galactosidase present in the culture during the experiment.
RESULTS Almost immediately upon addition of lactose, β-galactosidase began to accumulate, and its amount steadily increased. When lactose was removed, the enzyme did not disappear immediately (as would be the case if it were unstable). Instead, the amount of enzyme remained the same as when lactose was present. This result is expected only if β-galactosidase is a stable enzyme that is synthesized when lactose is added and stops being synthesized when lactose is removed.
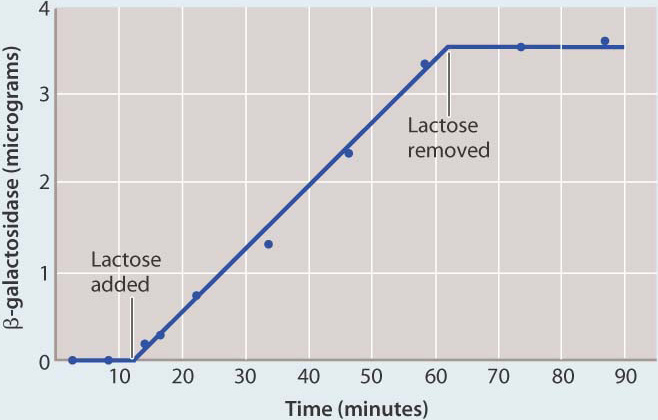
CONCLUSION Synthesis of β-galactosidase is turned on when lactose is added and turned off when lactose is removed, in support of the second hypothesis.
FOLLOW-UP WORK These results stimulated pioneering experiments that ultimately led to the discovery of the lactose operon and the mechanism of transcriptional regulation by a repressor protein.
SOURCE Monod, J. 1965. “From Enzymatic Adaption to Allosteric Transitions.” Nobel Prize lecture. http://nobelprize.org/nobel_prizes/medicine/laureates/1965/monod-lecture.htm.
To understand how the genes for lactose utilization are regulated, you need to know the main players, and these are shown in Fig. 19.15. The overall situation looks quite complicated, but when you take it apart and look at the individual pieces, it is in fact quite simple. Let us start with the two coding sequences on the right:
- lacZ is the gene (coding sequence) for β-galactosidase, which cleaves the lactose molecule into its glucose and galactose constituents.
- lacY is the gene (coding sequence) for lactose permease, which transports lactose from the external medium into the cell.
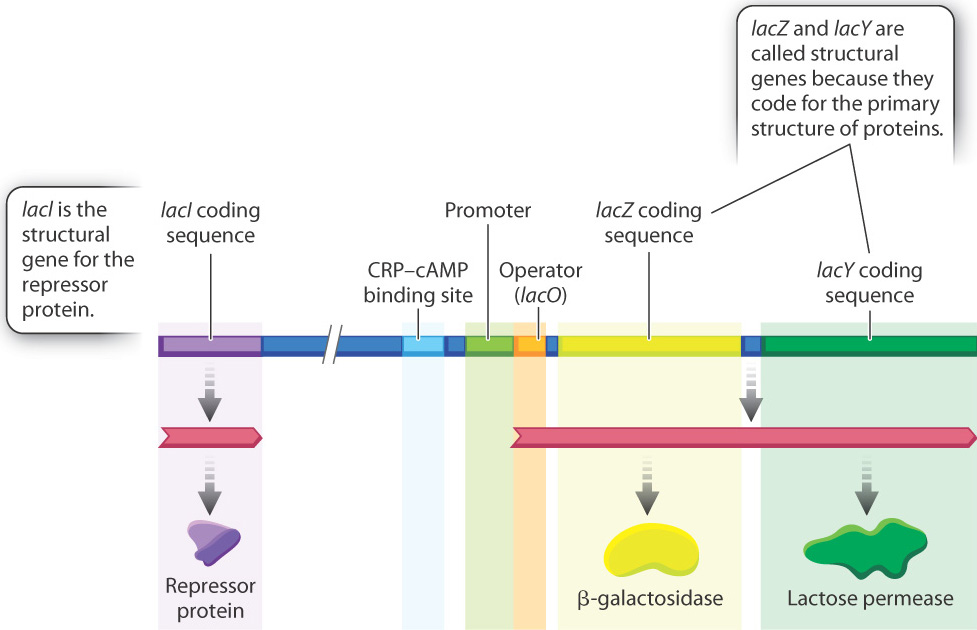
These genes are called structural genes because they code for the sequence of amino acids making up the primary structure of each protein. Bacteria that contain mutations in the lacZ gene or the lacY gene or both cannot utilize lactose as a source of energy. Without a functional product from lacY, lactose cannot enter the cell, and without a functional product from lacZ, lactose cannot be cleaved into its component sugars. Thus, β-galactosidase and permease are essential for the utilization of lactose for cell growth.
Regulation of the lacZ and lacY structural genes is controlled by the product of another structural gene, called lacI, which encodes a repressor protein. Located between lacI and lacZ are a series of regulatory sequences in the DNA that include a promoter, whose function is to recruit the RNA polymerase complex and initiate transcription, and an operator (lacO), which is the binding site for the repressor protein. Another regulatory region is a binding site for a protein called CRP, which is discussed later.
The kind of gene organization depicted in Fig. 19.15 is common in bacteria. Typically, a group of functionally related genes are located next to one another along the bacterial DNA, and when they are transcribed they are transcribed together into a single molecule of messenger RNA. Such an mRNA is called a polycistronic RNA (“cistron” is an old term for “coding sequence”). In Fig. 19.15, the polycistronic RNA includes the coding sequences for β-galactosidase and lactose permease. The region of DNA consisting of the promoter, the operator, and the coding sequence for the structural genes is called an operon.
Operons are found in bacteria and archaeons, whose cells can translate polycistronic mRNA molecules correctly because their ribosomes can initiate translation anywhere along an mRNA that contains a proper ribosome-binding site (Chapter 4). In a polycistronic mRNA, each of the coding sequences is preceded by a ribosome-binding site, so translation can be initiated there.
19.3.3 The repressor protein binds with the operator and prevents transcription, but not in the presence of lactose.
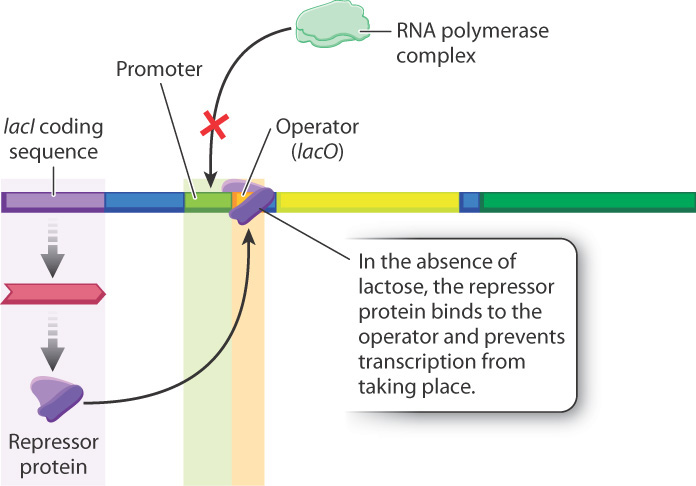
The lactose operon is negatively regulated by the repressor protein—that is, the structural genes of the lactose operon are always expressed unless the operon is turned off by a regulatory molecule, in this case the repressor. What the operon looks like in the absence of lactose is shown in Fig. 19.16. The lacI gene, encoding the repressor protein, is expressed constantly at a low level. The repressor protein binds with the operator (lacO), the RNA polymerase complex is not recruited, and transcription does not take place.
The configuration of the lactose operon in the presence of lactose is shown in Fig. 19.17. When lactose is present in the external medium, the repressor protein is unable to bind to the operator, RNA polymerase is recruited, and transcription occurs. In other words, lactose acts as an inducer since it prevents binding of the repressor protein. The inducer is not actually lactose itself, but rather an isomer of lactose called allolactose, which differs in the way the sugars are linked with each other. Allolactose is produced in small amounts whenever lactose is present in the cell.
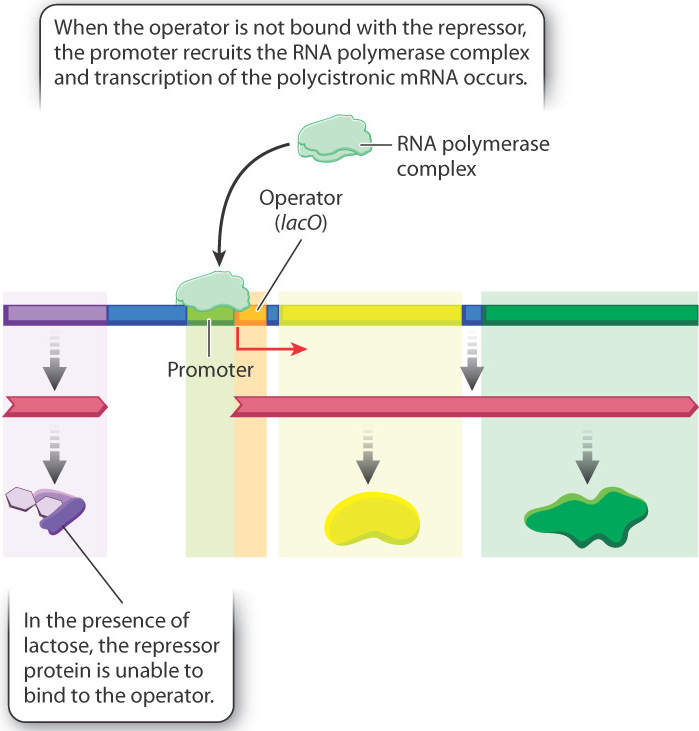
The binding of the inducer with the repressor protein results in an allosteric change in repressor structure that inhibits the protein’s ability to bind to the operator. The absence of repressor from the operator allows the RNA polymerase complex to be recruited to the promoter, and the polycistronic mRNA is produced. The resulting lactose permease allows the lactose to be transported into the cell on a large scale, and β-galactosidase cleaves the molecules to allow the constituents to be used as a source of energy and carbon. The lactose operon is therefore an example of negative regulation by the repressor, whose function is modulated by an inducer.
19.3.4 The function of the lactose operon was revealed by genetic studies.
Although the interactions shown in Fig. 19.16 and Fig. 19.17 have since been confirmed by direct biochemical studies, the inferences about how the repressor and operator work were originally drawn from studies of mutations (Fig. 19.18). As part of their investigation, Jacob and Monod identified bacterial mutants that expressed β-galactosidase and permease in the absence of lactose. The phenotype of a cell carrying such a mutation is said to be constitutive for production of the proteins. Constitutive expression means that it occurs continuously. The most common constitutive phenotype resulted from a mutation in the lacI gene that produced a defective repressor protein (Fig. 19.18a).
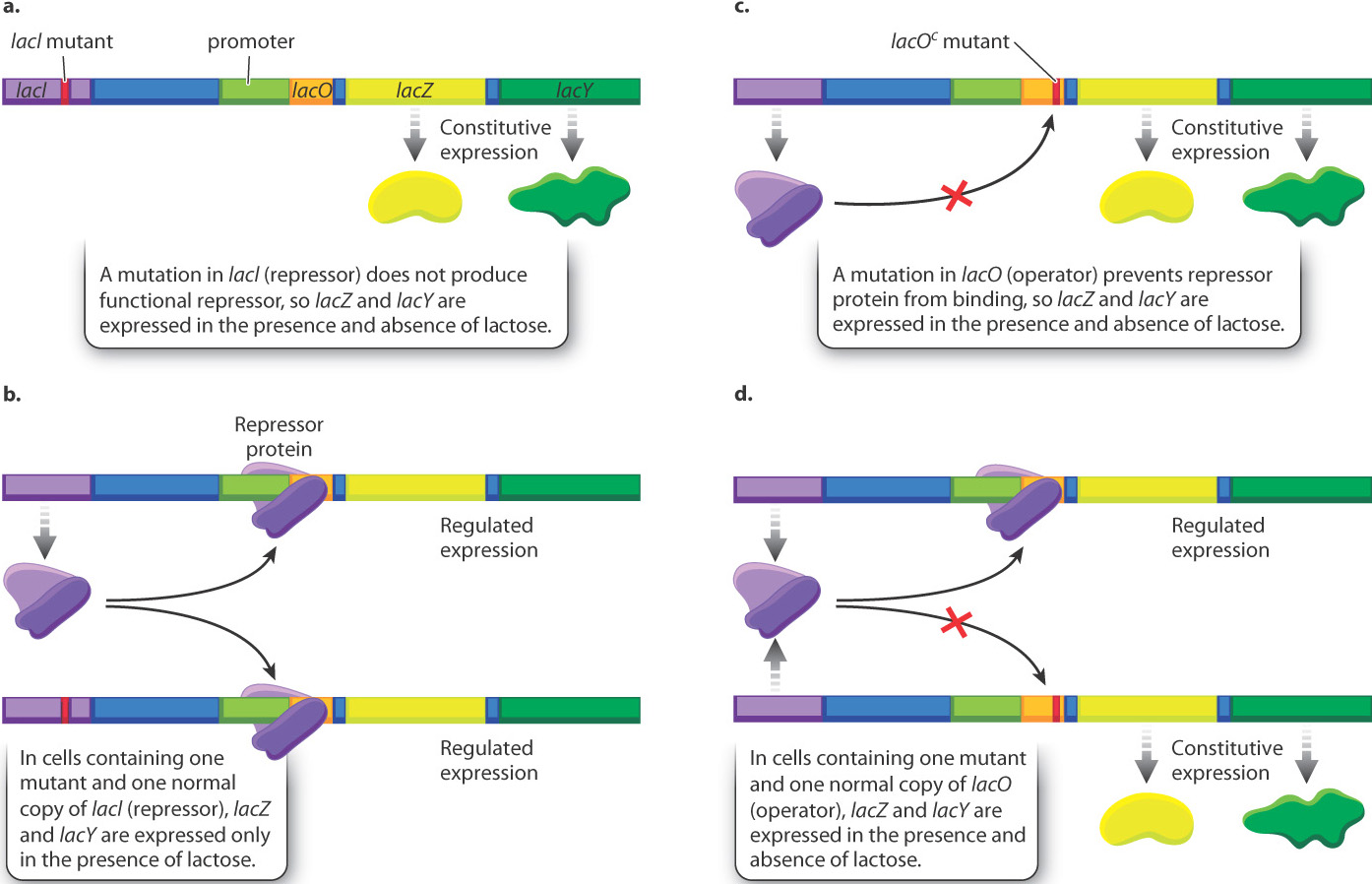
Jacob and Monod also did experiments in which E. coli contained not one but two lactose operons (Fig. 19.18b). In bacterial cells containing one mutant and one normal copy of lacI (repressor), gene expression was no longer constitutive but instead showed normal regulation. This finding is consistent with the idea that the lacI gene produces a diffusible protein since the normal repressor is able to bind to and repress transcription from both operons, not just the one that it is physically linked to.
A much less common class of constitutive mutants identified the operator (Fig. 19.18c). Genetic studies of the mutations in these cells showed that they were not located in the lacI gene that encodes the repressor but, rather, closer to the coding sequence of lacZ. The genetic element in which the mutations occurred was called the lactose operator (lacO), and the constitutive mutations were designated lacOc (“c” for “constitutive”). When two different lactose operons, one normal and one with lacOc, were in the same cell, the operon carrying lacOc was transcribed constitutively, even in the presence of normal repressor, because the repressor was unable to bind to the mutant operator site (Fig. 19.18d). “To explain this effect,” Jacob and Monod wrote, “it seems necessary to invoke a new type of genetic entity, called an ‘operator,’ which would be: (a) adjacent to the group of genes and would control their activity; and (b) would be sensitive to the repressor produced by a particular regulatory gene.”
Quick Check 4
Predict the consequence of a mutation in lacI (repressor gene) that produces repressor protein that is able to bind to the operator, but not able to bind allolactose.
19.3.5 The lactose operon is also positively regulated by CRP–cAMP.
Fig. 19.16 and Fig. 19.17 show how the ability of the repressor to bind with either the operator (in the absence of lactose) or with the inducer (in the presence of lactose) provides a simple and elegant way for the bacterial cell to transcribe the genes needed for lactose utilization only in the presence of lactose. The elucidation of these interactions was as far as the research tools used by Jacob and Monod could take them. Since the original experiments, the lactose operon has been studied in much greater detail and additional levels of regulation have been discovered.
One of these additional levels involves the CRP binding site shown in Fig. 19.15, which in Fig. 19.19 is occupied by a protein called the CRP–cAMP complex. The CRP–cAMP complex is a positive regulator of the lactose operon, which you will recall is a protein that activates gene expression upon binding to DNA. “CRP” stands for “cAMP receptor protein.” The role of CRP–cAMP is to provide another level of control of transcription that is more sensitive to the nutritional needs of the cell than the level of control provided by the presence or absence of lactose. E. coli can utilize many kinds of molecules as sources of energy. When more than one type of energy source is available in the environment, certain sources are used before others. For example, glucose is preferred to lactose, and lactose is preferred to glycerol. The CRP–cAMP complex helps regulate which compounds are utilized.
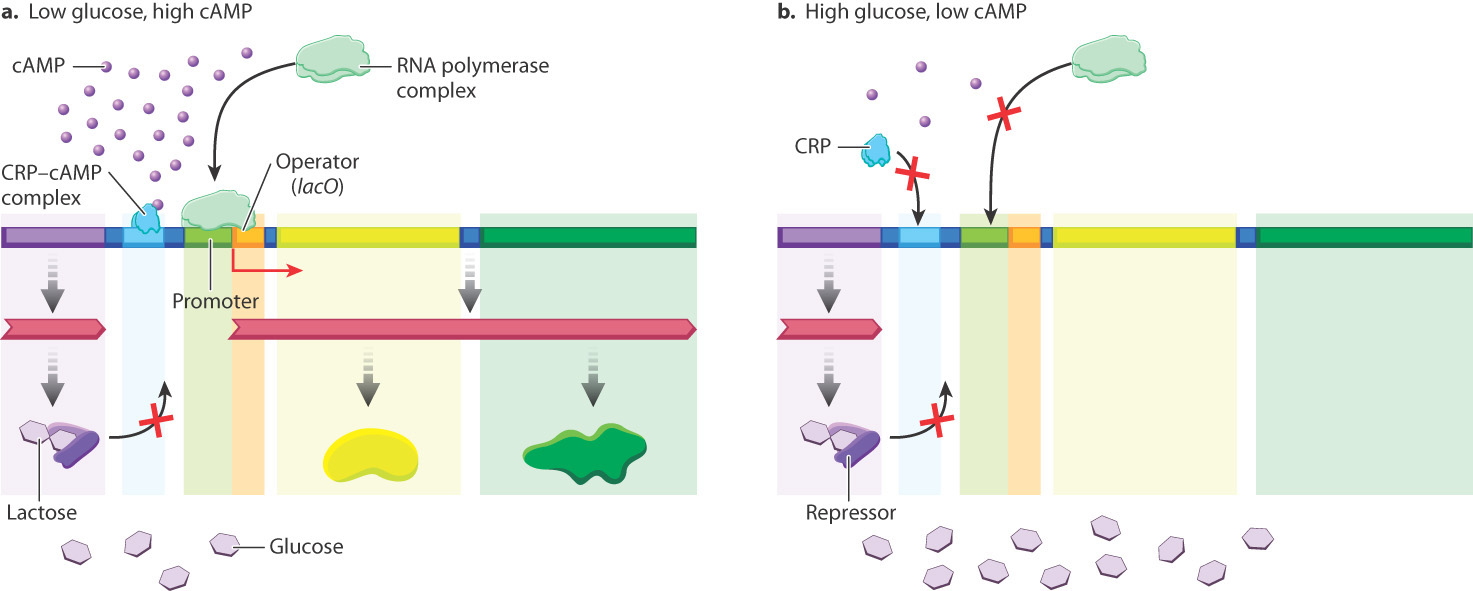
The concentration of the small molecule cAMP in the cell is a signal about the nutritional state of the cell. In the absence of glucose, cAMP levels are high, and cAMP binds to CRP, changing the shape of CRP so that it can bind DNA and activate transcription. In this way, cAMP is an allosteric activator of CRP, acting as described earlier. If lactose is present, the repressor can’t bind and the lactose operon is induced (Fig. 19.19a). If lactose is not present, the repressor is bound and the lactose operon is not transcribed even in the presence of the cAMP–CRP complex.
In the presence of glucose, cAMP levels are low, and the cAMP–CRP complex does not bind the lactose operon. As a result, even in the presence of lactose, the lactose operon is not transcribed to high levels (Fig. 19.19b). In this way, E. coli preferentially utilizes glucose when both glucose and lactose are present, and utilizes lactose only when glucose is depleted.
19.3.6 Transcriptional regulation determines the outcome of infection by a bacterial virus.
Transcriptional regulation has also been well studied in viruses. Bacterial cells are susceptible to infection by a variety of viruses known as bacteriophages (“bacteriophage” literally means “bacteria-eater,” and is often shortened to just “phage”). Among bacteriophages is a type that can undergo either of two fates when infecting a cell. The best known example is bacteriophage λ (lambda), which infects cells of E. coli. The possible fates of λ infection are illustrated in Fig. 19.20. Upon infection, the linear DNA of the phage genome is injected into the bacterial cell, and almost immediately the ends of the molecule join to form a circle. In normal cells growing in nutrient medium, the usual outcome of infection is the lytic pathway, shown at the left in Fig. 19.20. In the lytic pathway, the virus hijacks the cellular machinery to replicate the viral genome and produce viral proteins. After about an hour, the infected cell undergoes lysis and the cell bursts open to release a hundred or more progeny phage capable of infecting other bacterial cells.
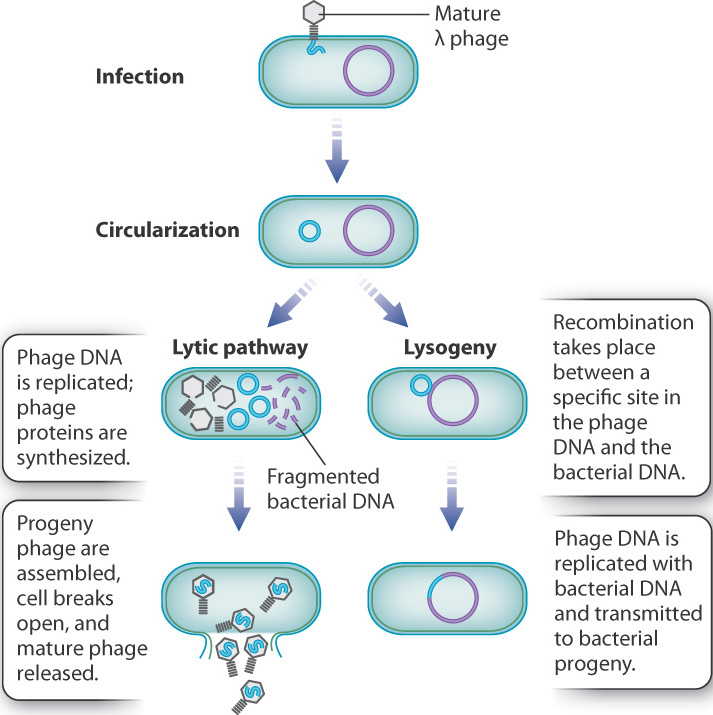
The alternative to the lytic pathway is lysogeny, shown at the right in Fig. 19.20. In lysogeny, the bacteriophage DNA and the bacterial DNA undergo a process of recombination at a specific site in both molecules, which results in a slightly enlarged bacterial DNA molecule that now includes the bacteriophage DNA. Lysogeny often takes place in cells growing in poor conditions. The relative sizes of the DNA molecules in Fig. 19.20 are not to scale. In reality, the length of the bacteriophage DNA is only about 1% of that of the bacterial DNA. When the bacteriophage genome is integrated in lysogeny, the only bacteriophage gene transcribed and translated is one that represses the transcription of other phage genes, preventing entry into the lytic pathway. The bacteriophage DNA is replicated along with the bacterial DNA and transmitted to the bacterial progeny when the cell divides. Under stress, such as exposure to ultraviolet light, recombination is reversed, freeing the bacterial genome and initiating the lytic pathway.
At the molecular level, the choice between the lytic and lysogenic pathways is determined by the positive and negative regulatory effects of a small number of bacteriophage proteins produced soon after infection. Fig. 19.21 shows the small region of the bacteriophage DNA in which the key interactions take place. Almost immediately after infection and circularization of the bacteriophage DNA, transcription takes place from the promoters PL and PR. The transcript from PR encodes the proteins cro and cII. The cro protein represses transcription from another promoter PM, which prevents transcription and synthesis of the protein cI. In normal cells growing in nutrient medium, proteases present in the bacterial cell degrade cII and prevent its accumulation. With cro protein preventing cI expression and cII protein unable to accumulate, transcription of bacteriophage genes in the lytic pathway takes place, including those genes needed for bacteriophage DNA replication, those encoding proteins in the bacteriophage head and tail, and, finally, those needed for lysis.
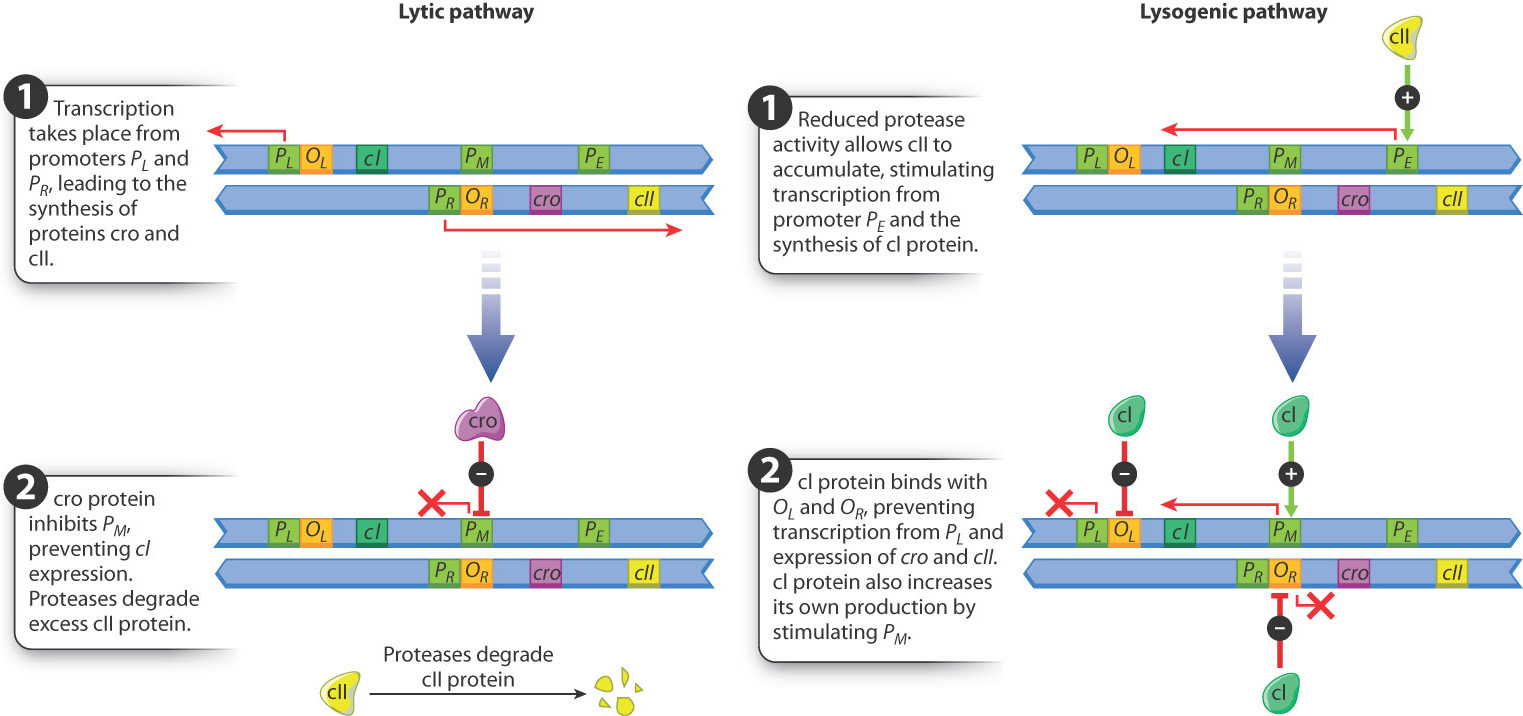
Alternatively, in bacterial cells growing in poor conditions, reduced protease activity allows cII protein to accumulate. When cII protein reaches a high enough level, it stimulates transcription from the promoter PE. The transcript from PE includes the coding sequence for cI protein, and the cI protein has three functions:
- It binds with the operator OR and prevents further expression of cro and cII.
- It stimulates transcription of its own coding sequence from the promoter PM, establishing a positive feedback loop that keeps the level of cI protein high.
- It binds with the operator OL and prevents further transcription from PL.
The result is that cI production shuts down transcription of all bacteriophage genes except its own gene, and this is the regulatory state that produces lysogeny. (The protein needed for recombination between the bacteriophage DNA and the bacterial DNA is produced by transcription from the PL promoter before it is shut down by cI.) When cells that have undergone lysogeny are exposed to ultraviolet light or certain other stresses, the cI protein is degraded. In this case, cro and cII are produced again, and the lytic pathway follows.
Regulation of the lytic pathway and lysogeny works to the advantage of bacteriophage λ, but the process is not as simple and elegant as something an engineer might design. That is because biological systems are not engineered, they evolve. Regulatory mechanisms are built up over time by the selection of successive mutations. Each evolutionary step refines the regulation in such a way as to be better adapted to the environment than it was before. Each successive step occurs only because it increases survival and reproduction.
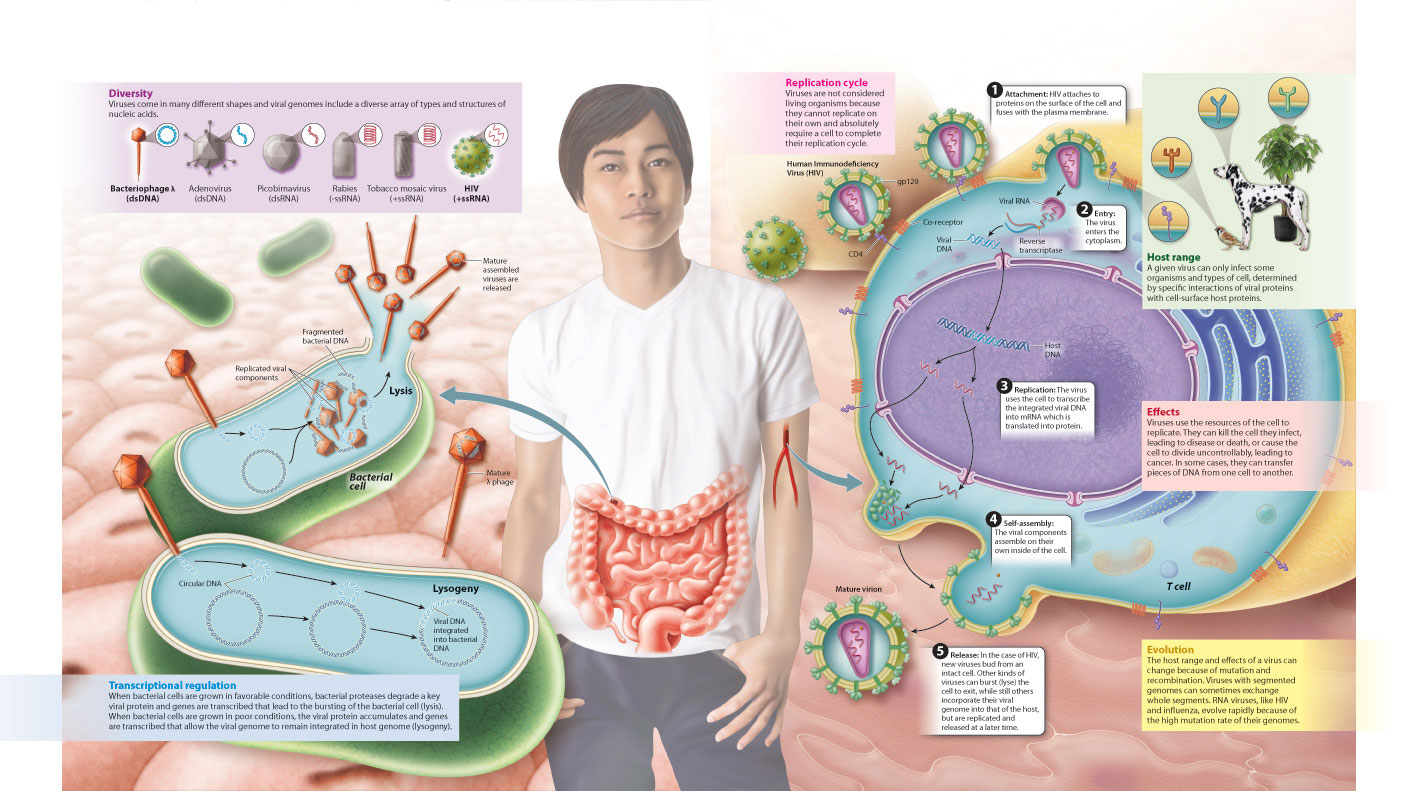
We summarize these two alternative outcomes of infection by a bacterial virus, and the earlier discussions of viral diversity, replication, host range, and effects on a cell in Fig. 19.22 on the following page.
19.3.7 Visual Synthesis: Virus: A Genome in Need of a Cell
Integrating concepts from Chapters 11, 13, and 19.
Click the image below to view an enlarged version in a new window.