20.1 GENETIC PROGRAMS OF DEVELOPMENT
Genetic programs and computer programs have a lot in common. Computer code is written as a linear string of letters, which corresponds to the sequence of nucleotides in genomic DNA. Once a computer program is initiated, it automatically runs and performs its coded task. Small mistakes in the code, analogous to mutations, can have big consequences and even cause the program to crash.
The analogy between genetic programs and computer programs has an important limitation. Computer programs, designed by humans, are consciously written, whereas genetic programs evolve. The genetically encoded developmental programs of all living organisms emerged over hundreds of millions of years through mutation and natural selection. These developmental programs changed gradually through time, persisting only if they produced organisms that could successfully survive and reproduce in the existing environment. Here, we explore the genetic program of development—that is, the genetic instructions that lead from a single fertilized egg to a complex multicellular organism.
20.1.1 The fertilized egg is a totipotent cell.
In all sexually reproducing organisms, the fertilized egg is special because of its developmental potential. The fertilized egg is said to be totipotent, which means that it can give rise to a complete organism. In mammals, the egg also forms the membranes that surround and support the developing embryo (Chapter 42).
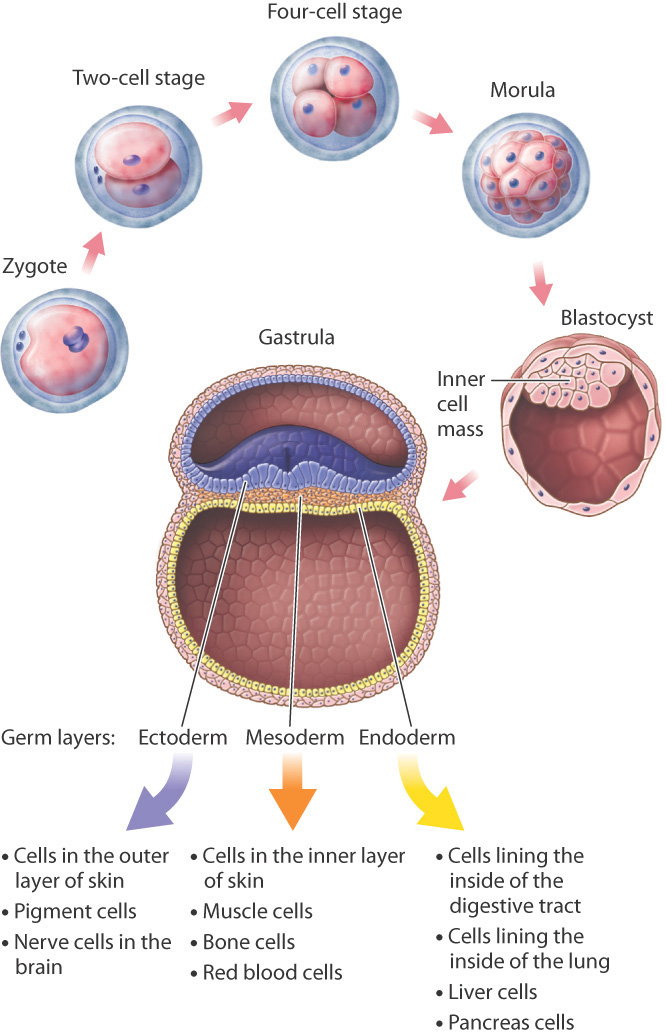
Development begins with fertilization, which in human females normally occurs in one of the two fallopian tubes stretching from the upper corners of the uterus to the ovaries on either side, where the tubes flare out with tiny fingerlike projections to catch the egg released at ovulation. The fertilized egg, or zygote, travels down the fallopian tube toward the uterus, undergoing mitotic cell division as it moves along. One cell becomes two, two become four, four become eight, eight become sixteen, and so on, with all the cells contained within the egg’s outer membrane (Fig. 20.1). This clump of cells, called the morula, reaches the uterus about 4 or 5 days after fertilization.
These early cell divisions are different from mitotic cell division later in life because the cells do not grow between divisions; they merely replicate their chromosomes and divide again. The result is that the cytoplasm of the egg is partitioned into smaller and smaller packages, the new cells all bunched together inside the gelatinous envelope that covers the developing embryo.
Cell division continues in the morula until there are a few thousand cells. The cells then begin to move in relation to one another, pushing against and expanding the membrane that encloses them and rearranging themselves to form a hollow sphere called a blastocyst (Fig. 20.1). In one region of the inner wall of the blastocyst, there is a group of cells known as the inner cell mass, from which the body of the embryo develops. The wall of the blastocyst forms several membranes that envelop and support the developing embryo. Once the blastocyst forms, 5 or 6 days after fertilization, it implants in the uterine wall. From implantation onward, the cells in the blastocyst approximately double their size with each cell division. This is the first great trial of the embryo, which can no longer draw on the cytoplasm in the egg produced by the mother. To survive, the cells of the embryo must now manufacture their own cytoplasm.
Once implanted in the uterine wall, the multiplying cells of the inner cell mass reorganize to form a gastrula. It is at this stage that the three germ layers are established (Fig. 20.1). Germ layers are sheets of cells that include the ectoderm, mesoderm, and endoderm and that differentiate further into specialized cells. Those formed from the ectoderm include epithelial cells and pigment cells in the skin and nerve cells in the brain; cells from the mesoderm include cells that make up the inner layer of the skin, muscle cells, and red blood cells; and cells formed from the endoderm include cells of the lining of the digestive tract and lung, as well as liver cells and pancreas cells.
20.1.2 Cellular differentiation increasingly restricts alternative fates.
At each successive stage in development, as the cells become differentiated they lose the potential to develop into any kind of cell. The fertilized egg is totipotent because it can differentiate into both the inner cell mass and supporting membranes, and eventually into an entire organism. The cells of the inner cell mass, called embryonic stem cells, are pluripotent because they are able to give rise to any of the three germ layers, and therefore to any cell of the body. However, pluripotent cells cannot on their own give rise to an entire organism, as a totipotent cell can. Cells further along in differentiation are multipotent; these cells can form a limited number of types of specialized cell. Totipotent, pluripotent, and multipotent cells are all considered stem cells, cells that are capable of differentiating into different cell types.
Quick Check 1
From what you know about embryonic development, do you think that a cell from the inner cell mass or one from the ectoderm has more developmental potential?
Why do differentiating cells increasingly lose their developmental potential? One hypothesis focuses on gene regulation. When cells become committed to a particular developmental pathway, genes no longer needed are turned off (that is, repressed) and are difficult to turn on again. Another hypothesis is genome reduction: As cells become differentiated, they delete the DNA for genes they no longer need.
These hypotheses can be distinguished by an experiment in which differentiated cells are reprogrammed to mimic earlier states. If loss of developmental potential is due to gene regulation, then cells could be reprogrammed to become pluripotent or multipotent. If loss of developmental potential is due to genome reduction, then differentiated cells could not be reprogrammed to become pluripotent or multipotent.
British developmental biologist John Gurdon carried out such experiments in the early 1960s (Fig. 20.2). Gurdon used a procedure called nuclear transfer, in which a hollow glass needle is used to insert the nucleus of a cell into the cytoplasm of an egg whose own nucleus has been destroyed or removed. Previous nuclear transfer experiments had been carried out in the leopard frog, Rana pipiens. Whereas nuclei from pluripotent or multipotent cells could often be reprogrammed to develop into normal tadpoles, attempts with nuclei from fully differentiated cells failed.
FIG. 20.2How do stem cells lose their ability to differentiate into any cell type?
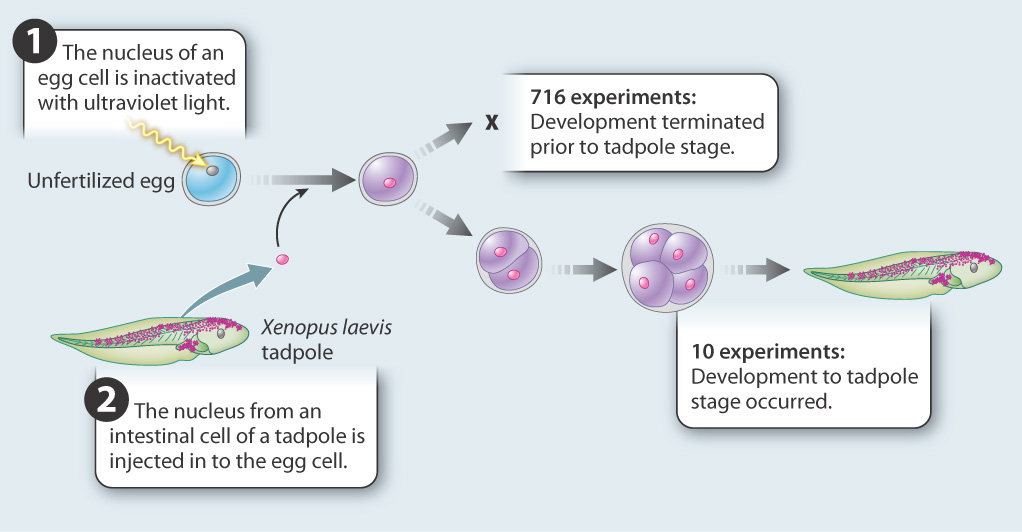
BACKGROUND During differentiation, cells become progressively more specialized and restricted in their fates. Early studies left it unclear whether cell differentiation occurs because of changes in gene expression, or whether cell differentiation results from loss of genes.
HYPOTHESES One hypothesis is that differentiation occurs as a result of changes in gene expression. A second hypothesis is that differentiation occurs as a result of genome reduction, in which genes that are not needed are deleted.
EXPERIMENT John Gurdon carried out experiments in the amphibian Xenopus laevis to test these hypotheses. He transferred nuclei from differentiated cells into unfertilized eggs whose nuclei had been inactivated with ultraviolet light. If differentiation is due to changes in gene expression, then the differentiated nucleus should be able to reprogram itself in the egg cytoplasm and differentiate again into all the cells of a tadpole. If differentiation is accompanied by loss of genes, then differentiation is irreversible and development will not proceed.
RESULTS The experiment was carried out 726 times. In 716 cases, development did not occur; in 10, development proceeded normally.
CONCLUSION Although the experiment succeeded in only 10 of 726 attempts, it showed that the nucleus of an intestinal cell and the cytoplasm of the unfertilized egg are able to support complete development of a normal animal. This result allows us to reject the hypothesis that differentiation occurs by the loss of genes. Differentiated cells must contain a complete genome. The first hypothesis—that cells become differentiated as a result of changes in gene expression—was supported. But, because of the small number of successes in reprogramming, additional experiments were needed to validate the conclusions.
FOLLOW-UP WORK This work was controversial. Some critics argued that the successful experiments resulted from a small number of undifferentiated cells present in intestinal epithelium. Others accepted the conclusion but expressed misgivings about possible applications to humans. Later experiments that succeeded in cloning mammals from fully differentiated cells confirmed the original conclusion.
SOURCE Gurdon, J. B. 1962. “The Developmental Capacity of Nuclei Taken from Intestinal Epithelium Cells of Feeding tadpoles.” Journal of Embryology & Experimental Morphology 10: 622–640.
Gurdon tried the experiments in a different organism, the clawed toad Xenopus laevis, and demonstrated that nuclei from fully differentiated intestinal cells could be reprogrammed to support normal development of the tadpole (Fig. 20.2). Only 10 of 726 experiments succeeded, but this was sufficient to show that intestinal cell nuclei still contained a complete Xenopus genome. In other words, his findings supported the first hypothesis—all of the same genes are present in intestinal cells as in early embryonic cells, but some of the genes are turned off, or repressed, during development.
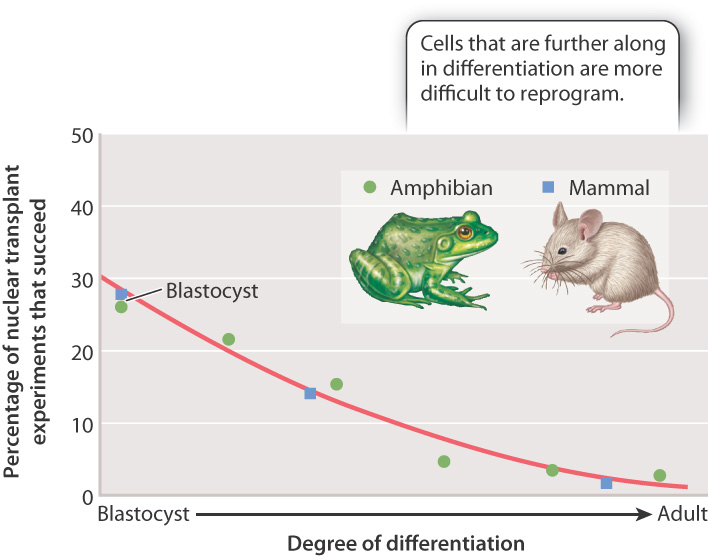
Fig. 20.3 summarizes the results of many nuclear transfer experiments carried out in mammals and amphibians. The percentage of reprogramming experiments that fail increases as cells differentiate. The best chance of success is to use pluripotent nuclei from cells in the blastocyst (or its amphibian equivalent, the blastula). However, even some experiments using nuclei from fully differentiated cells have been successful.
When nuclear transfer succeeds, the result is a clone—an individual that carries an exact copy of the nuclear genome of another individual. In this case, the new individual shares the same genome as that of the individual from which the donor nucleus was obtained. (The mitochondrial DNA is not from the nuclear donor, but from the donor of the egg cytoplasm.) The first mammalian clone was a lamb called Dolly (Fig. 20.4a), born in 1996. She was produced from the transfer of the nucleus of a cell in the mammary gland of a sheep, and was the only successful birth among 277 nuclear transfers. Successful cloning in sheep soon led to cloning in cattle, pigs, and goats.

The first household pet to be cloned was a kitten named CopyCat (Fig. 20.4b), born in 2001 and derived from a differentiated ovarian cell. CopyCat was the only success among 87 tries. As shown in Fig. 20.4b, the cat from which the donor nucleus was obtained was a calico, but CopyCat herself was not, even though the two cats are clones of each other. The reason for their different appearance has to do with X-inactivation, discussed in Chapter 19. Recall that the mottled orange and black calico pattern results from random inactivation of one of the two X chromosomes during development. The lack of a calico pattern in CopyCat implies that the X chromosomes in the transferred nucleus did not “reset” as they do in normal embryos. Instead, the inactive X in the donor nucleus remained inactive in all the cells in the clone. Hence, while CopyCat and her mother share the same nuclear genome, the genes were not expressed in the same way because of irreversible epigenetic regulation in the donor nucleus.
Quick Check 2
X-inactivation results in two clones of cells differing in the genes expressed. Can you think of other reasons why two genetically identical individuals might look different from each other?
Case 3 You, From A to T: Your Personal Genome
20.1.3 Can cells with your personal genome be reprogrammed for new therapies?
Stem cells play a prominent role in regenerative medicine, which aims to use the natural processes of cell growth and development to replace diseased or damaged tissues. Stem cells are already used in bone marrow transplantation and may someday be used to treat Parkinson’s disease, Alzheimer’s disease, heart failure, certain types of diabetes, severe burns and wounds, and spinal cord injury.
At first it seemed as though the use of embryonic stem cells gave the greatest promise for regenerative medicine because of their pluripotency. This approach proved ethically controversial because obtaining embryonic stem cells requires the destruction of human blastocysts. A major breakthrough took place in 2006 when Japanese scientists demonstrated that adult cells can be reprogrammed by activation of just a handful of genes, most of them encoding transcription factors or chromatin proteins. The reprogrammed cells were pluripotent and were therefore called induced pluripotent cells (iPS cells).
The success rate was only about one iPS cell per thousand, and the genetic engineering technique required the use of viruses that can sometimes cause cancer. Nevertheless, the result was regarded as spectacular. Other researchers soon found other genes that could be used for reprogramming adult cells into pluripotent or multipotent stem cells, and still other investigators developed virus-free methods for delivering the genes. In recent years, researchers have even discovered small organic molecules that can reprogram adult cells.
This kind of reprogramming opens the door to personalized stem cell therapies. The goal is to create stem cells derived from the adult cells of the individual patient. Since these cells contain the patient’s own genome, problems with tissue rejection are minimized or eliminated (Chapter 43). There remains much to learn before therapeutic use of induced stem cells becomes routine. Researchers will face challenges such as increasing the efficiency of reprogramming, verifying that reprogramming is complete, making sure that the reprogrammed cells are not prone to cancer, and demonstrating that the reprogrammed cells differentiate as they should. Nevertheless, researchers hope that someday soon your own cells containing your personal genome could be reprogrammed to restore cells or organs damaged by disease or accident.