20.5 CELL SIGNALING IN DEVELOPMENT
As we have seen in the discussion of stem cells, differentiated cells can be reprogrammed by the action of only a few key genes or small organic molecules. In some cases, the processes that push differentiation in a forward direction are also quite simple. An important example is signal transduction, in which an extracellular molecule acts as a signal to activate a membrane protein that in turn activates molecules inside the cell that control differentiation (Chapter 9). The signaling molecule is called the ligand and the membrane protein that it activates is called the receptor. The following example shows how a simple receptor–ligand pair can have profound effects not only on the differentiation of the cell that carries the receptor but also on its neighbors.
20.5.1 A signaling molecule can cause multiple responses in the cell.
Pioneering experiments on signal transduction in development were carried out in the soil nematode Caenorhabditis elegans (Fig. 20.23). This worm is a favored model organism for developmental studies because of its small size (the adult is about 1 millimeter long), short generation time (2½ days from egg to sexually mature adult), and stereotyped process of development. (A stereotyped process is one that is always the same.) The adult animal consists of exactly 959 somatic (body) cells, and the cells’ patterns of division, migration, differentiation, and morphogenesis are identical in each individual C. elegans. The adult is typically a hermaphrodite that produces both male and female reproductive cells. The eggs are fertilized internally by sperm, and, after undergoing five or six cell divisions, the eggs are laid through an organ known as the vulva (Fig. 20.23).
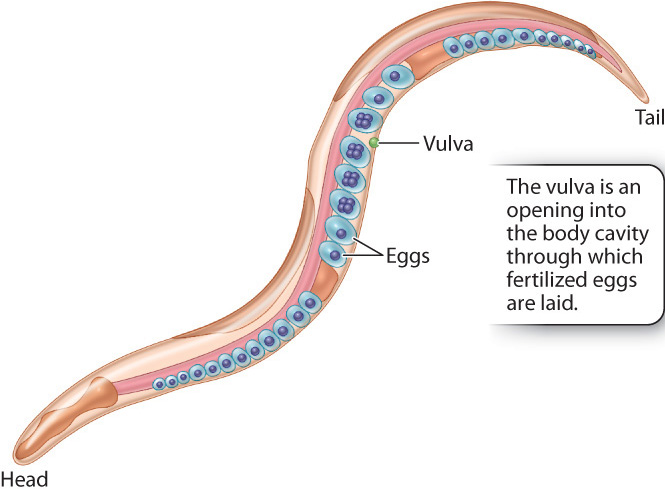
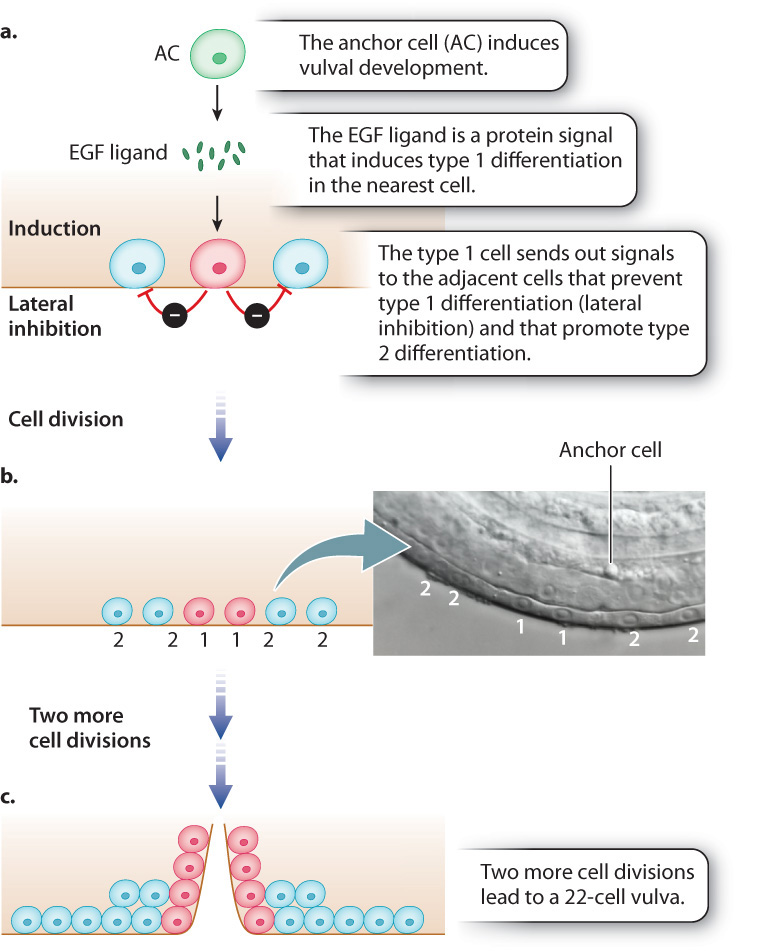
Vulva development is relatively simple and therefore amenable to experimental studies. The entire structure arises from only three cells that undergo either of two types of differentiation and divide to produce a vulva consisting of exactly 22 cells. Cells resulting from type 1 differentiation form the opening of the vulva, and cells resulting from type 2 differentiation form a supporting structure around the vulval opening. In each of the two types of differentiation, different genes are activated or repressed, resulting in differentiated cells with different functions.
How each of the three original cells, called progenitor cells, differentiates is determined by its position in the developing worm (Fig. 20.24). The fate of the progenitor cells is determined by their proximity to another cell, called the anchor cell (AC in Fig. 20.24a), which secretes a protein called epidermal growth factor (EGF) that binds to and activates a transmembrane EGF receptor (Chapter 9). The progenitor cell closest to the anchor cell receives the most amount of signal, and upon activation of its EGF receptor the progenitor cell carries out three functions:
- It activates the genes for differentiation into a type 1 cell (Fig. 20.24a)
- It prevents the adjacent cells from differentiating as type 1 (a process called lateral inhibition).
- It induces the adjacent cells to differentiate as type 2 cells.
20.5.2 Developmental signals are amplified and expanded.
How can a single ligand–receptor pair cause so many changes in gene expression that it determines the pathway of differentiation not only of the cell itself but also of its neighbors? Fig. 20.25 shows the mechanisms in simplified form. On the left is the progenitor cell nearest the anchor cell, which receives the strongest EGF signal. Activation of the EGF receptor by the ligand initiates a process of signal transduction in the cytoplasm, in which the signal is transmitted from one protein to the next by means of phosphorylation, which amplifies the signal at each stage (Chapter 9). The result of signal transduction is that a set of transcription factors is activated.
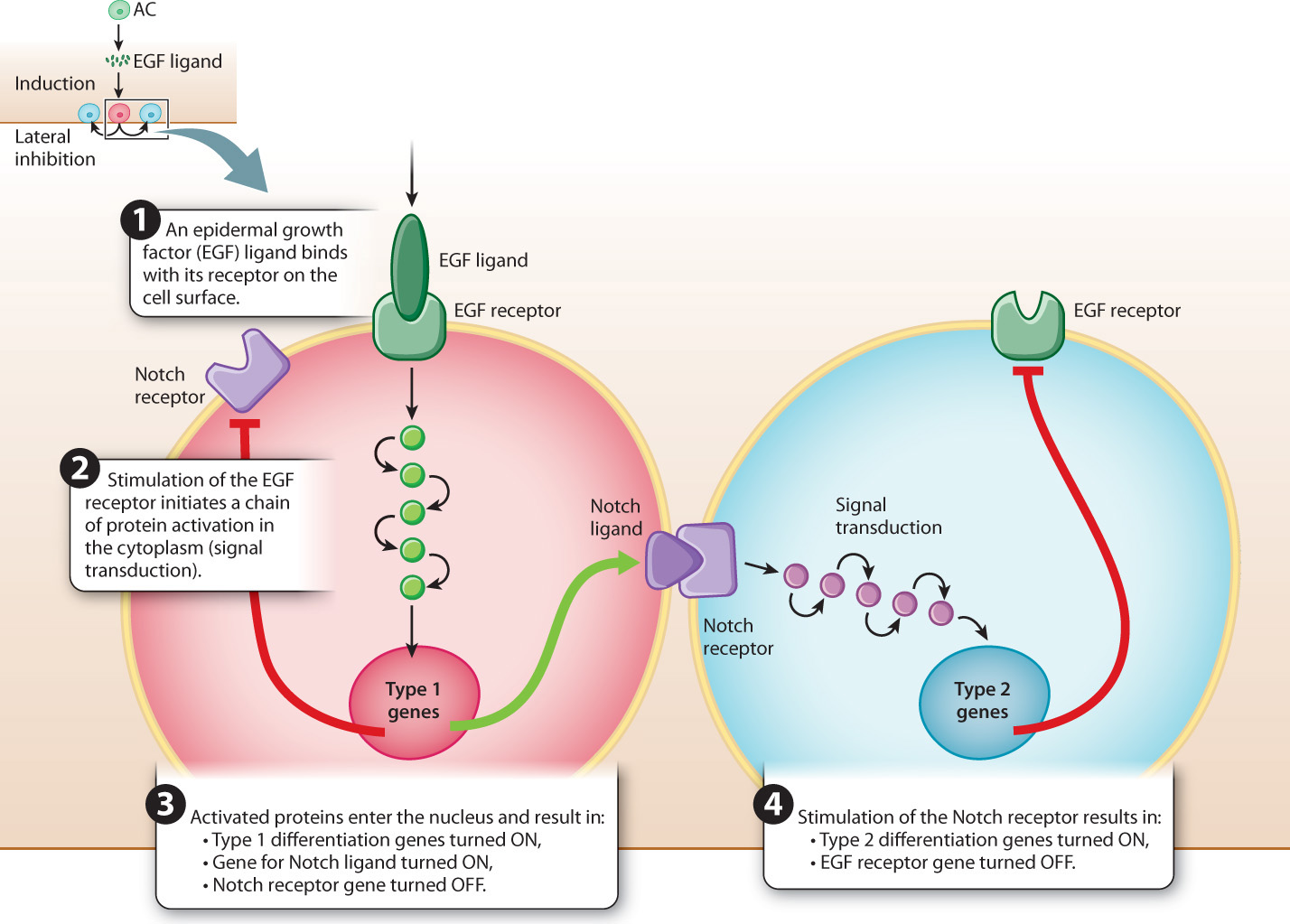
In the nucleus, the transcription factors activate transcription of genes for type 1 differentiation. The transcription factors also activate transcription of genes that produce another type of protein ligand, called Notch. The Notch ligand is a transmembrane protein that activates Notch receptors in the neighboring cells. Activation of the Notch receptor in these cells activates another signal transduction cascade, which results in transcription of the genes for type 2 differentiation. The cascade started by the binding of Notch also activates transcription of other genes whose products inhibit the EGF receptor. Inhibiting the EGF receptor in type 2 cells prevents EGF from eliciting a type 1 response in the type 2 cell. In addition, at the same time that the Type 1 cell produces the Notch ligand, it produces proteins that inhibit its own Notch receptors, and this prevents Notch from initiating a type 2 response in the type 1 cell.
While vulva development in nematodes is a fairly simple example of the importance of ligand–receptor signaling in development, EGF and Notch ligands and their receptors are found in virtually all animals. They are among dozens of ligand–receptor pairs that have evolved as signaling mechanisms to regulate processes in cellular metabolism and development. Humans have several distinct but related gene families of EGF and Notch ligands and receptors. Human EGF is important in cell survival, proliferation, and differentiation. EGF functions in the differentiation and repair of multiple types of cells in the skin; it is present in all body fluids and helps regulate rapid metabolic responses to changing conditions. Human Notch ligands are involved in development of the nervous and immune systems as well as heart, pancreas, and bone. Abnormalities in EGF or Notch signaling are associated with many different types of cancer.
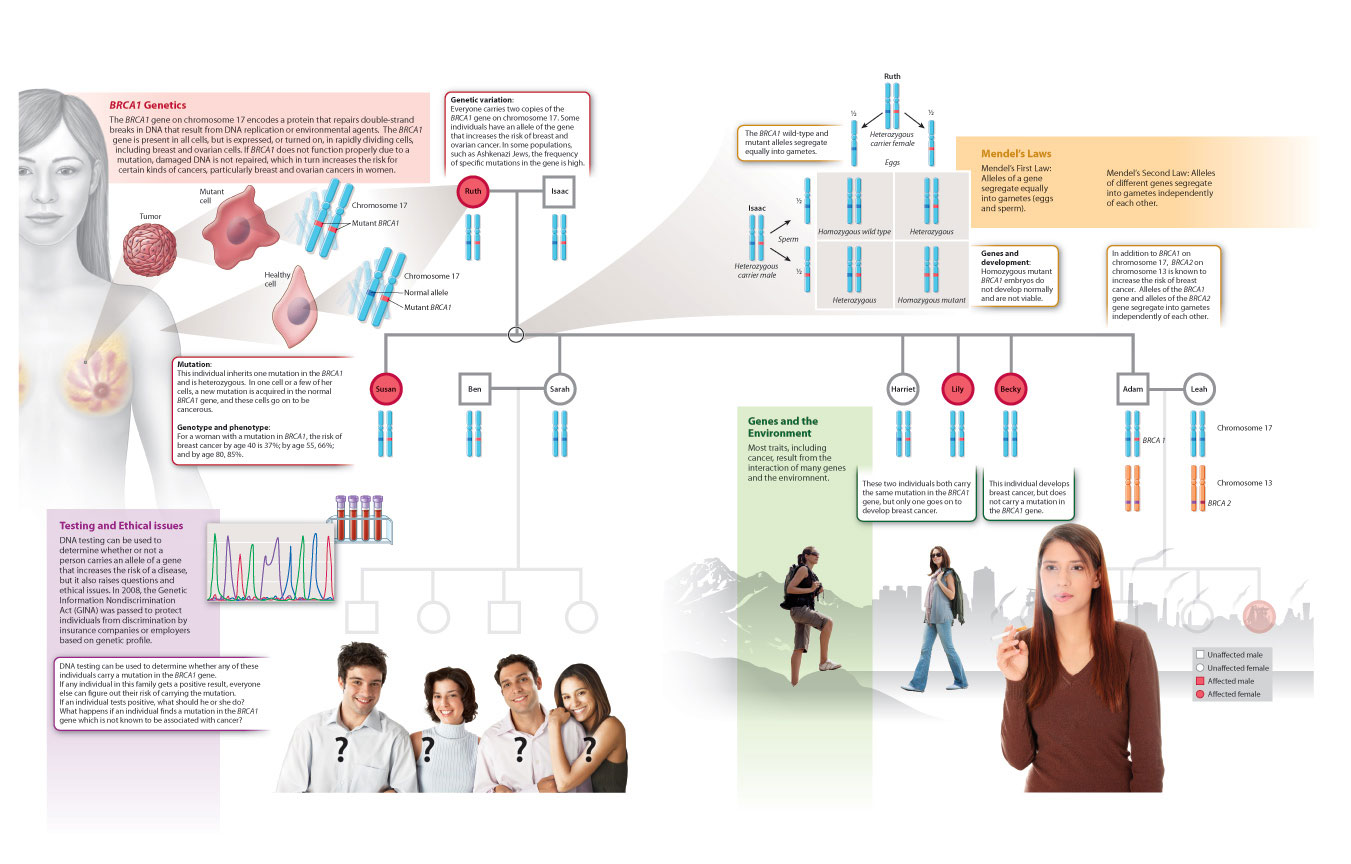
Therefore, just as we saw in our discussion of eye and flower development, the molecular players involved in development are often evolutionarily conserved across a wide range of organisms. This is true even of genes that we typically associate with disease, such as BRCA1 and BRCA2 and their link with cancer. These genes not only play a role in cell cycle control in the adult, but also in early development in many organisms. In fact, although heterozygous mutations in these genes predispose to breast and ovarian cancers in humans, homozygous mutations are lethal in early embryonic stages. We focus on the BRCA1 gene to summarize key concepts about DNA replication, mutation, genetic variation, inheritance, gene regulation, and development in Fig. 20.26.
Visual Synthesis: Genetic Variation and Inheritance
Integrating concepts from Chapters 12-20
Click the image below to view an enlarged version in a new window.