24.2 AFRICAN ORIGINS
For a long time, it was argued that modern humans, Homo sapiens, derive from the Homo ergaster populations that spread around the world starting about 2 million years ago. This idea is called the multiregional hypothesis of human origins because it implies that different Homo ergaster populations throughout the Old World evolved in parallel, with some limited gene flow among them, to each produce modern H. sapiens populations. In short, modern human traits evolved convergently in multiple populations (Chapter 23). This idea was overturned in 1987 by another study from Allan Wilson’s laboratory, which instead suggested that modern humans arose much more recently from Homo ergaster descendants (sometimes called Homo heidelbergensis; see Fig 24.6) in Africa, about 200,000 years ago. This newer idea is the out-of-Africa hypothesis of human origins.
Quick Check 2
What do the multiregional and out-of-Africa hypotheses predict about the age of the common ancestor of all humans living today?
24.2.1 Studies of mitochondrial DNA reveal that modern humans evolved in Africa.
To test these two hypotheses about human origins, Rebecca Cann, a student in Allan Wilson’s laboratory, chose to analyze DNA sequences to reconstruct the human family tree. Specifically, she studied sequences of a segment of mitochondrial DNA from people living around the world (Fig. 24.8).
FIG. 24.8When and where did the most recent common ancestor of all living humans live?
BACKGROUND With the availability of DNA sequence analysis tools, it became possible to investigate human prehistory by comparing the DNA of living people from different populations.
HYPOTHESIS The multiregional hypothesis of human origins suggests that our most recent common ancestor was living at the time Homo ergaster populations first left Africa, about 2 million years ago.
METHOD Rebecca Cann compared mitochondrial DNA (mtDNA) sequences from 147 people from around the world. This approach required a substantial amount of mtDNA from each individual, which she acquired by collecting placentas from women after childbirth. Instead of sequencing the mtDNA, Cann inferred differences among sequences by digesting the mtDNA with different restriction enzymes, each of which cuts DNA at a specific sequence (Chapter 12). If the sequence is present, the enzyme cuts. If any base in the recognition site of the restriction enzyme has changed in an individual, the enzyme does not cut. The resulting fragments were then separated using gel electrophoresis. By using 12 different enzymes, Cann was able to assay a reasonable proportion of all the mtDNA sequence variation present in the sample.
Here, we see a sample dataset for four people and a chimpanzee. There are seven varying restriction sites:
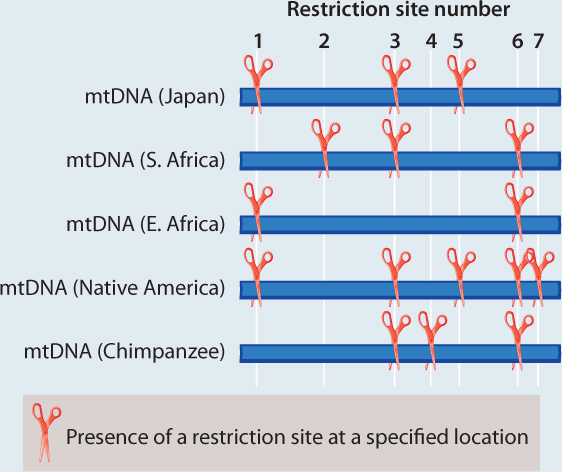
ANALYSIS The data were converted into a family tree by using shared derived characters, described in Chapter 23. By mapping the pattern of changes in this way, the phylogenetic relationships of the mtDNA sequences can be reconstructed. For example, the derived “cut” state at restriction site 1 shared by the East African, Japanese, and Native American sequences implies that the three groups had a common ancestor in which the mutation that created the new restriction site occurred.
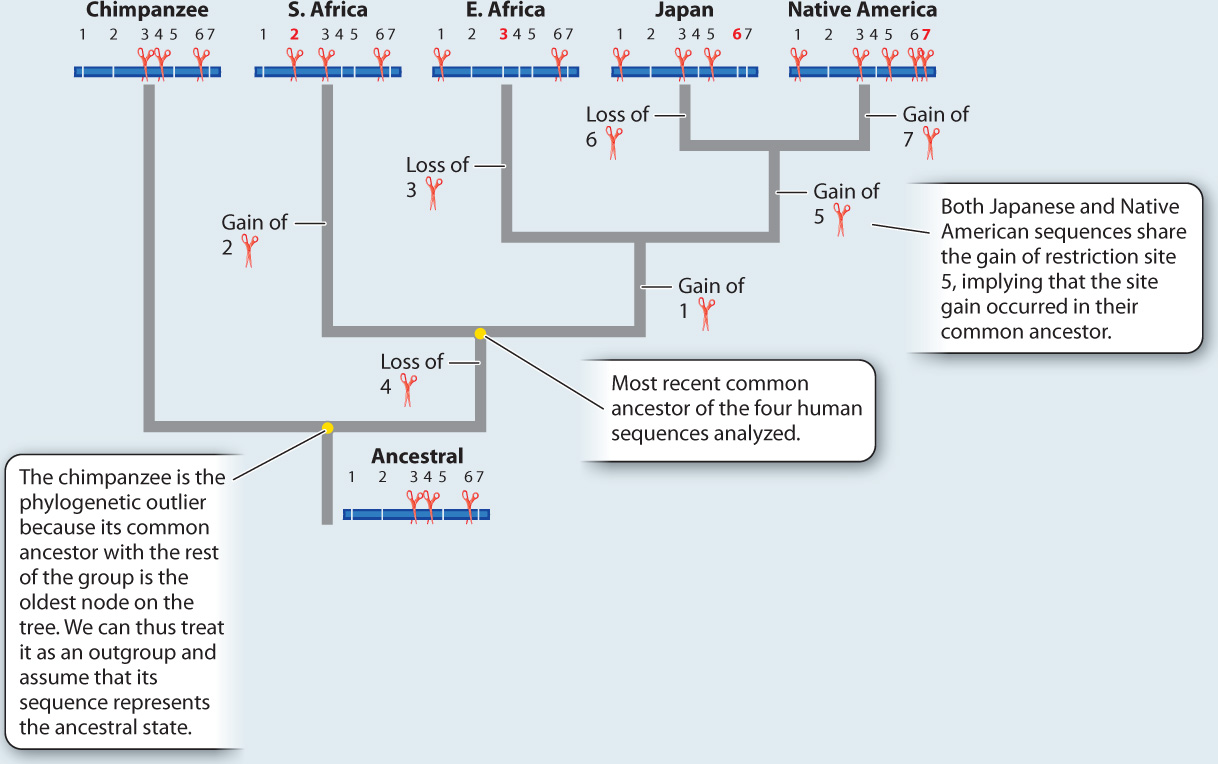
RESULTS AND CONCLUSION A simplified version of Cann’s phylogenetic tree based on mtDNA is shown below. Modern humans arose relatively recently in Africa, and their most common ancestor lived about 200,000 years ago. The multiregional hypothesis is rejected. Also, the data revealed that all non-Africans derive from within the African family tree, implying that H. sapiens left Africa relatively recently. The Cann study gave rise to the out-of-Africa theory of human origins.
FOLLOW-UP WORK This study set the stage for an explosion in genetic studies of human prehistory that used the same approach of comparing sequences in order to identify migration patterns. Today, with molecular tools vastly more powerful than those available to Cann, studies of the distribution of genetic variation are giving us a detailed pattern, even on relatively local scales, of demographic events.
SOURCE Cann, R. L., M. Stoneking, and A. C. Wilson. 1987. “Mitochondrial DNA and Human Evolution.” Nature 325:31–36.
Mitochondrial DNA (mtDNA) is a small circle of DNA, about 17,000 base pairs long, found in every mitochondrion (Chapter 17). Cann chose to study mtDNA for several reasons. Although a typical cell contains a single nucleus with just two copies of nuclear DNA, each cell has many mitochondria, each carrying multiple copies of mtDNA. Thus, mtDNA is much more abundant than nuclear DNA and therefore easier to extract. Most important, however, is its mode of inheritance. All your mtDNA is inherited from your mother in the egg she produces because sperm do not contribute mitochondria to the zygote. This means that there is no opportunity for genetic recombination between different mtDNA molecules, so the only way in which sequence variation can arise is through mutation. In nuclear DNA, by contrast, differences between two sequences can be introduced through both mutation and recombination. Recombination obscures genealogical relationships because it mixes segments of DNA with different evolutionary histories.
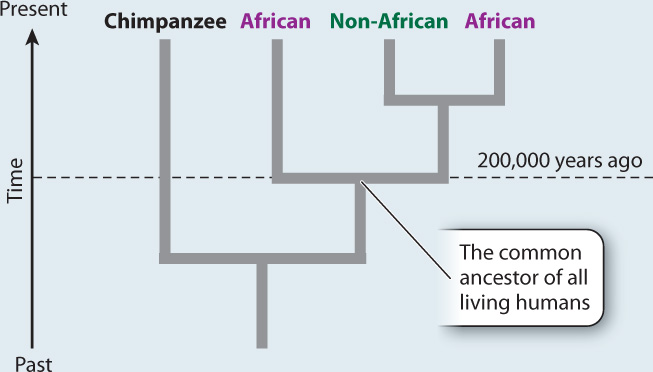
Mitochondrial DNA sequences offered Cann a clear advantage. With sequence information from the mtDNA of 147 people from around the world, Cann reconstructed the human family tree (Fig. 24.8). The tree contained two major surprises. First, the two deepest branches—the ones that come off the tree earliest in time—are African. All non-Africans are branches off the African tree. The implication is that Homo sapiens evolved in Africa and only afterward did populations migrate out of Africa and become established elsewhere. This finding contradicted the expectations of the multiregional theory, which predicted that Homo sapiens evolved independently in different locations throughout the Old World.
Second, the tree is remarkably shallow, that is, even the most distantly related modern humans have a relatively recent common ancestor. By calibrating the rate at which mutations occur in mtDNA, Cann was able to estimate the time back to the common ancestor of all modern humans as about 200,000 years. Subsequent analyses have somewhat refined this estimate, but the key message has not changed: The data contradict the multiregional theory, which predicted a number closer to 2 million years.
Two hundred thousand years ago is 10 times more recent than 2 million years ago, so this change represents a revolution in our understanding of human prehistory. But surely it is risky to make such major claims on the basis of a single study. Maybe there’s something peculiar about mtDNA, and it doesn’t offer an accurate picture of the human past. To find independent evidence for a recent origin of modern humans in Africa, another dataset was required. One such dataset comes from studies of the Y chromosome, another segment of human DNA that does not undergo recombination (Chapter 17). When an approach similar to Cann’s was used to reconstruct the human family tree using Y chromosome DNA sequences, the result was completely in agreement with the mtDNA result: The human family is young, and it arose in Africa.
24.2.2 Neanderthals disappear from the fossil record as modern humans appear but have contributed to the modern human gene pool.
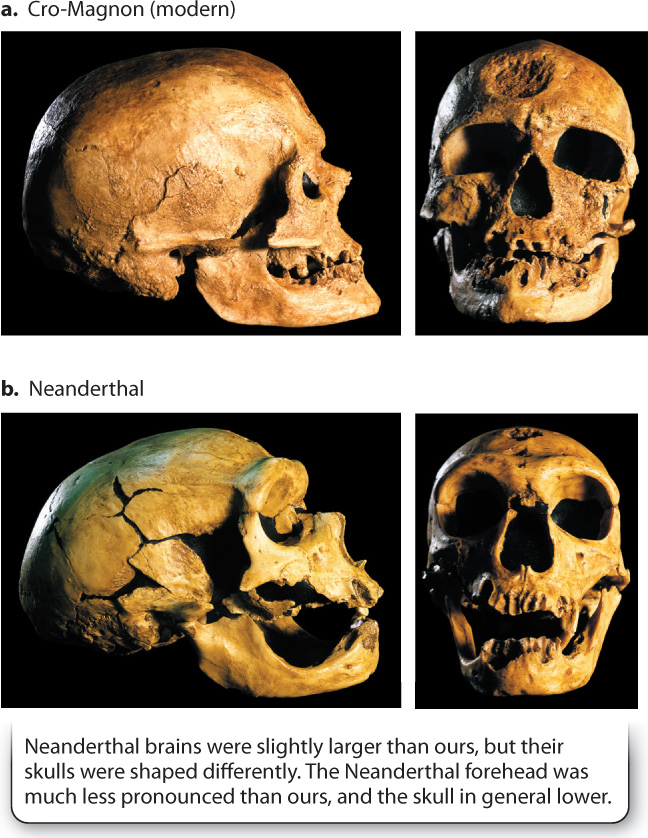
If we exclude H. floresiensis, the last of the nonmodern humans were the Neanderthals (Fig. 24.9), who lived in Europe and Western Asia until about 30,000 years ago. What happened? Were they eliminated by the first population of Homo sapiens to arrive in Europe (a group known as Cro-Magnon for the site in France from which specimens were first described)? Or did they interbreed with the Cro-Magnons, and, if so, does that mean that Neanderthals should be included among our direct ancestors? The controversy surrounding this question flares up from time to time when paleontologists find specimens that have anatomical features intermediate between the heavyset Neanderthal form and the lighter human one.
In 1997, we thought we had a clear answer. Matthias Krings working in Svante Pääbo’s lab in Germany extracted intact stretches of mtDNA from 30,000-year-old Neanderthal material. If Neanderthals had interbred with our ancestors, we would expect to see evidence of their genetic input in the form of Neanderthal mtDNA sequences in the modern human gene pool. However, the Neanderthal sequence was strikingly different from that of modern humans. That we see nothing even close to the Neanderthal mtDNA sequence in modern humans strongly suggested that Neanderthals did not interbreed with our ancestors (Fig. 24.10a).
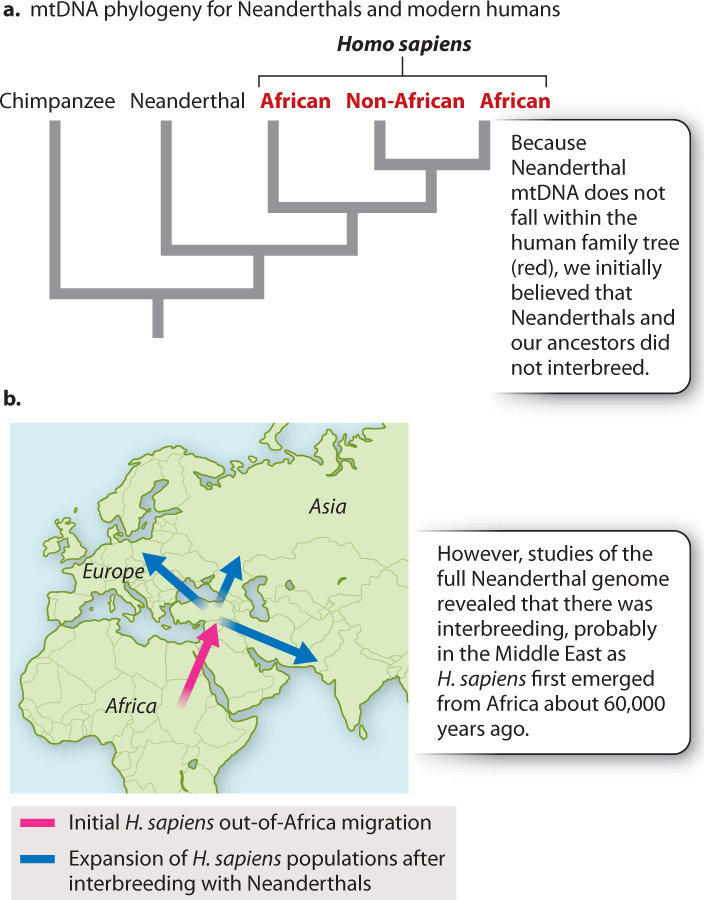
This conclusion, however, has been reversed following remarkable technological advances that permitted Pääbo and others to sequence the entire Neanderthal genome (rather than just its mtDNA). Careful population genetic analysis revealed that our ancestors did interbreed with Neanderthals, and that 1% to 4% of the genome of every non-African is Neanderthal-derived. A scenario emerged: As populations of our ancestors headed from Africa into the Middle East, they encountered Neanderthals, and it was then, before modern humans had spread out across the world, that interbreeding took place (Fig. 24.10b).
How can we reconcile these two apparently contradictory results? The mtDNA study indicates there was no genetic input from Neanderthals in modern humans, but the whole-genome study suggests that in fact there was. One possible solution lies in the difference in patterns of transmission between mtDNA and genomic material (Chapter 17). Recall that mtDNA is maternally inherited because sperm do not contribute mitochondrial material. Your mtDNA, whether you are male or female, is derived solely from your mother. One possibility is that the Neanderthal mtDNA lineage has been lost through genetic drift (Chapter 21). An alternative is that the discrepancy in the ancient DNA stems from a sex-based difference in interbreeding. If Neanderthal females did not interbreed with our ancestors, Neanderthal mtDNA would not have entered the modern human gene pool. If only male Neanderthals interbred with our ancestors, we would expect exactly the pattern we observe: no Neanderthal mtDNA in modern humans, but Neanderthal genomic DNA in the modern human gene pool.
It is likely that our interpretation of these events will always be speculative. The basic result, however, forces us to reimagine the ancestry of a large portion of the human population. Every non-African on the planet is part Neanderthal, albeit a very small part.
Quick Check 3
Explain how genetic data suggest that only male Neanderthals interbred with our ancestors.