26.5 THE DIVERSITY OF ARCHAEA
We have already noted that Archaea and Bacteria are both prokaryotic, but that they differ in their membrane and wall composition, molecular mechanisms for transcription and translation, and many other biological features (see Table 26.1). As a result, the two groups tend to thrive in very different environments. Many archaeons tolerate environmental extremes such as heat and acidity. In fact, for many environmental conditions, archaeons define the known limits of life. What do these disparate environments have in common? One proposal is that archaeons thrive in environments where the energy available to fuel cell activities is limited—where there is no light, or a limited abundance of fuels and oxidants for chemoautotrophy or respiration, or both. That is, archaeons commonly live where energy resources are too low to support bacteria and eukaryotes.
Bacteria and Archaea diverged from their last common ancestor more than 3 billion years ago. Just as Bacteria have evolved a phylogenetic diversity that defies easy categorization, Archaea also include many distinct types of organism. Many microbiologists recognize three major divisions of Archaea, called the Crenarchaeota, Euryarchaeota, and Thaumarchaeota, but a few poorly known groups may indicate further major branches within the domain (for example, the Korarchaeota and Nanoarchaeota noted in Fig. 26.17).
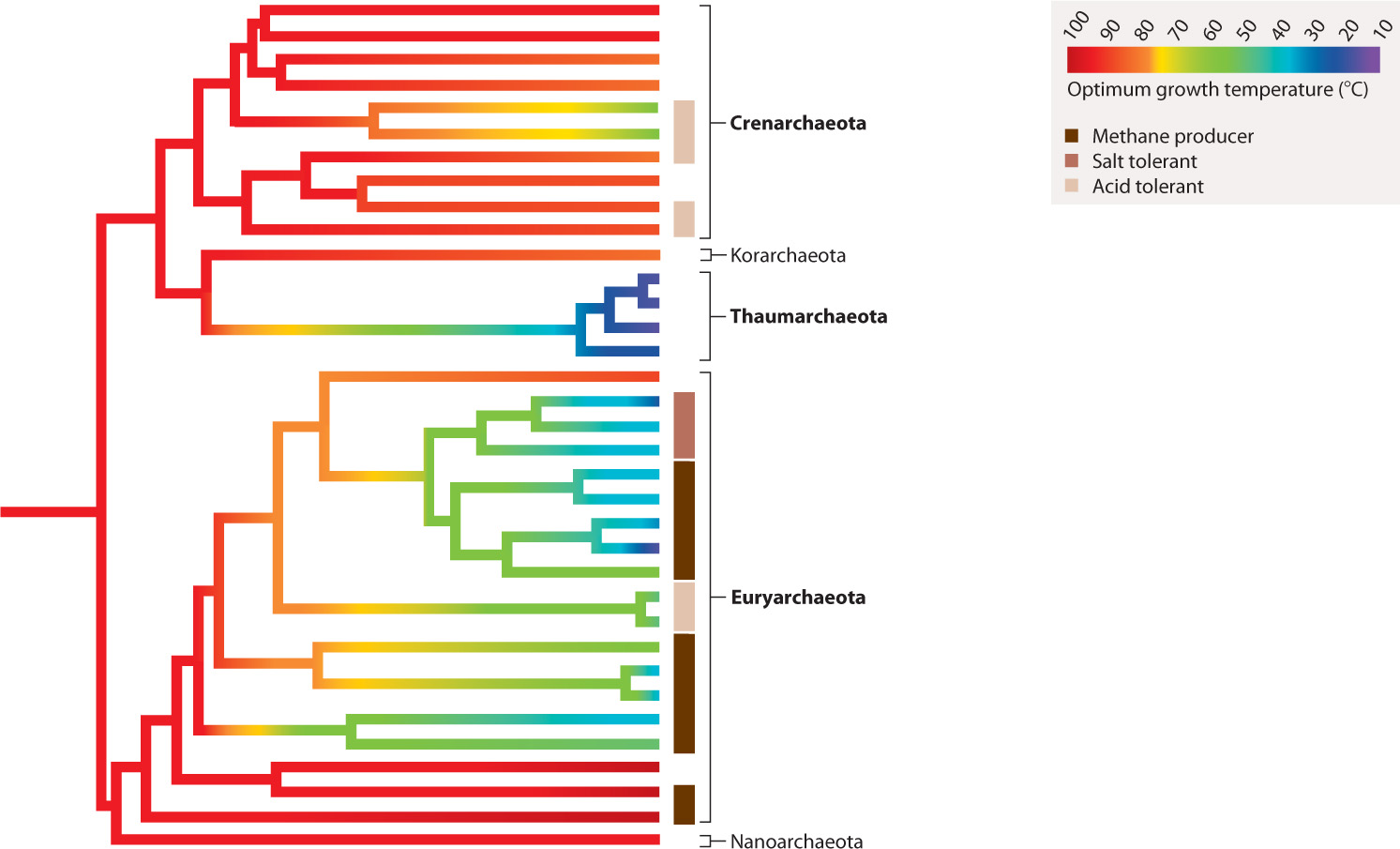
26.5.1 The archaeal tree has anaerobic, hyperthermophilic organisms near its base.
Archaeons found near the base of the Crenarchaeota and Euryarchaeota branches exhibit a remarkable ability to grow and reproduce at temperatures of 80 °C or more (Fig. 26.17). Called hyperthermophiles, these organisms do not simply tolerate high temperature, they require it. At present, the world record for high temperature growth is held by Methanopyrus kandleri, which has been shown to grow at 122°C among hydrogen-and CO2-rich hydrothermal vents on the seafloor beneath the Gulf of California. Most of these heat-loving archaeons are anaerobic, living as anaerobic respirers or as chemoautotrophs that obtain energy from the oxidation of hydrogen gas.
Why do anaerobic hyperthermophiles sit on the lowest branches of the archaeal tree? Many biologists believe that these features characterized the first Archaea and therefore imply that the Archaea evolved in hot environments with no oxygen. As we discussed in Chapter 23, geologists agree that oxygen gas was not present on the early Earth, consistent with the phylogenetic inference that early Archaea were anaerobic. A hot environment for early Archaea can be understood in two ways. Either the entire ocean was hot—for which there is little evidence—or Archaea first evolved in hot springs where local supplies of hydrogen and metals enabled them to live as chemoautotrophs. Because some versions of the bacterial tree also show hyperthermophilic species on their lowest branches, it has been suggested that the last common ancestor of all living organisms lived in a hot environment.
Quick Check 5
If early branches on the bacterial and archaeal trees are dominated by hyperthermophilic microorganisms, does this mean that the early oceans were very hot?
26.5.2 The Crenarchaeota and Euryarchaeota both include acid-loving microorganisms.
In many parts of the globe where humans mine coal or metal ores, abandoned mines fill up with very acidic water. Called acid mine drainage, this water has been acidified through the oxidation of pyrite (FeS2) and other sulfide minerals to produce sulfuric acid (H2SO4). The pH of these waters is commonly around 1 to 2, and can actually fall below 0. To us, acid mine drainage is an environmental catastrophe, but a number of archaeons thrive in it (Fig. 26.18). Modified protein and membrane structures enable them to tolerate their harsh surroundings, making it possible to use sulfur compounds in the environment for both chemoautrophic and heterotrophic growth. What is the limit to growth at low pH? Picrophilus torridus, a euryarchaeote archaeon, grows optimally at 60°C and in environments with a pH of 0.7—the pH of battery acid. It is capable of growth in environments down to pH 0—a record, as far as we know. In fact, Picrophilus torridus requires an acidic environment. It will not grow in acid rainwater because the pH is too high.

26.5.3 Euryarchaeote archaeons include heat-loving, methane-producing, and salt-loving microorganisms.
Some euryarchaeote archaeons, like Picrophilus torridus, are thermophilic—heat-loving. Many other euryarchaeotes, uniquely among organisms, generate natural gas, or methane (CH4), as a by-product of fermentation or chemoautotrophic metabolism. Methanogenic archaeons play an important role in the carbon cycle, recycling organic molecules back to simple gases in environments where the supplies of electron acceptors for respiration (oxygen gas, sulfate ion, ferric iron) are limited. Methanogenic archaeons are prominent in peats and lake bottoms where sulfate levels are low and bacterial sulfate reduction is therefore limited. Most methanogenic organisms live in soil or sediments, but some live in the rumens (a specialized chamber in the gut) of cows, helping to maximize the energy yield from ingested grass. Methane doesn’t last long in the atmosphere—it is quickly oxidized to carbon dioxide. However, the steady supply of methane generated by archaeons contributes significantly to the greenhouse properties of the atmosphere. For this reason, biologists are interested in how methanogenic archaeons will respond to 21st-century climate change.
A related group of euryarchaeote archaeons illustrates the limits of tolerance to another environmental condition—salinity, or saltiness. Halophilic (salt-loving) archaeons are photoheterotrophs that use the protein bacteriorhodopsin to absorb energy from sunlight (see Fig. 26.6). They live in waters that are salty enough to precipitate table salt (NaCl). As is correspondingly true of extreme acidophiles, these extreme halophiles require high salt conditions and cannot live in dilute water.
26.5.4 Thaumarchaeota may be the most abundant cells in the oceans.
Another surprise of environmental sequencing has been the discovery that the immense volumes of ocean beneath the surface waters contain vast numbers of archaeons that have not yet been cultured in the lab. In the deep ocean, these organisms may be nearly as abundant as the combined number of all bacteria on and below the surface. Recently segregated as the Thaumarchaeota, these archaeons are chemoautotrophs, deriving energy from the oxidation of ammonia (Fig. 26.19). Thus, like the discovery of SAR11, the discovery of marine thaumarchaeotes makes it clear that before the still-emerging age of environmental genetics and genomics, we simply didn’t have a very good idea of which microorganisms lived where and did what in the oceans. Fortunately, that situation is changing rapidly.
FIG. 26.19How abundant are archaeons in the oceans?
BACKGROUND Early exploration of microbial diversity in the oceans revealed that archaeons are tremendously abundant. Just how abundant are they, and what metabolisms do they employ to obtain energy and carbon?
METHOD Scientists sampled seawater throughout the depth of the ocean at a test site in the northern Pacific Ocean. To the samples, they added molecular tags bound to fluorescent molecules that are visible under the microscope. The tags were RNA sequences that bind to small-subunit ribosomal RNA genes known to be useful in identifying different types of bacteria and archaeons. In this way, separate fluorescent markers were attached to thaumarchaeote archaeons, euryarchaeote archaeons, and bacteria.
RESULTS By counting the cells marked by different fluorescent tags in seawater samples, the biologists showed that bacteria dominate microbial communities in near-surface seawater, but that thaumarchaeotes are as numerous as bacteria in deeper waters (Fig. 26.19a).
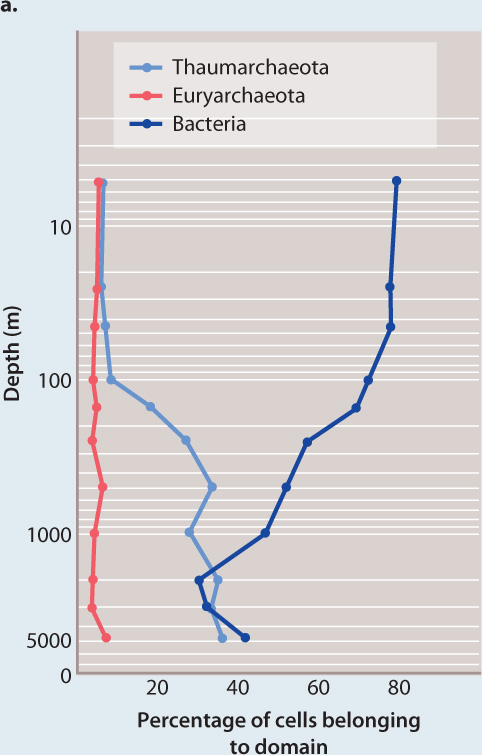
Surveys of cell abundance through a depth profile of the Pacific Ocean show that bacteria dominate cell numbers near the surface, but thaumarchaeotes make up about 40% of all cells in deeper waters.
CONCLUSION Marine thaumarchaeota, unknown in 1990, are now known to be among the most abundant organisms in the oceans.
FOLLOW-UP WORK Archaea are among the most abundant of all cells in the world’s oceans. How do these cells live? Microbial samples from water known to be sites of nitrification (that is, the conversion of ammonia to nitrite or nitrate) contained an abundance of thaumarchaeote cells. The high numbers of thaumarchaeotes from these communities supported the hypothesis that they are nitrifiers—the higher the abundance of thaumarchaeote cells (Fig. 26.19b, dark blue line), the higher the amount of nitrite (Fig. 26.19b, light blue line). Thaumarchaeotes were grown in pure culture and shown to grow by consuming ammonia (Fig. 26.19b, red line). In other words, they oxidize ammonia to provide the ATP and reducing power needed to incorporate CO2 into organic molecules. Therefore, they play a major role in the marine nitrogen cycle, thriving where sources of carbon and energy for other types of metabolism are scarce.
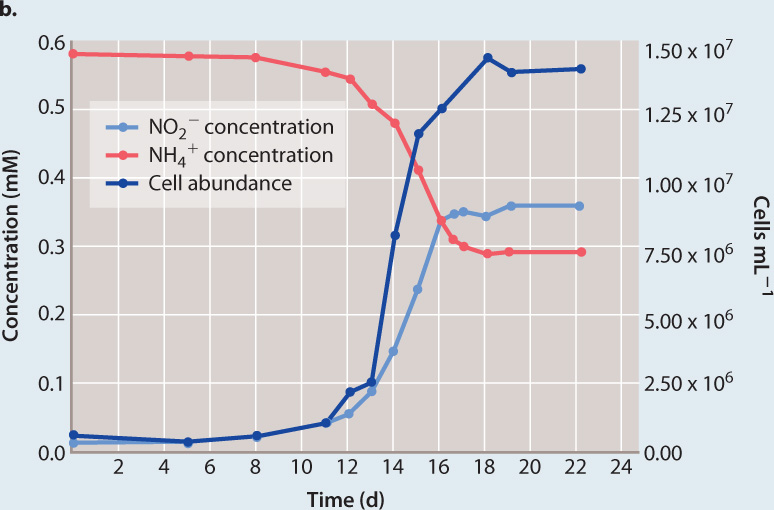
Growth of a marine thaumarchaeote in a medium containing ammonium chloride and bicarbonate as the only sources of energy and carbon, respectively. As cell number increased, ammonia (the ammonium ion NH4+) was increasingly converted to nitrite (NO2−), supporting the hypothesis that these cells are ammonia-oxidizing chemoautotrophs.
SOURCES Karner, M. B., E. F. DeLong, and D. M. Karl. 2001. “Archaeal Dominance in the Mesopelagic Zone of the Pacific Ocean.” Nature 409:507–510; Könneke, K., A. E. Bernhard, J. R. de la Torres, C. B. Walter, J. B. Waterbury, and D. A. Stahl. 2005. “Isolation of an Autotrophic Ammonia-Oxidizing Marine Archaeon.” Nature 437:543–546.