26.6 THE EVOLUTIONARY HISTORY OF PROKARYOTES
On the tree of life, the plants and animals of our familiar existence populate only the most recent branches. For most of Earth history, life was entirely microbial. Perhaps surprisingly given their small size, Bacteria and Archaea have left a fossil record that allows paleontologists to reconstruct aspects of life’s early evolution. Sedimentary rocks preserve microscopic fossils of ancient bacteria, including the photosynthetic cyanobacteria (Fig. 26.20). Limestones and related rocks preserve stromatolites, layered structures that record sediment accumulation by microbial communities (Fig. 26.21). Some especially well preserved ancient rocks also contain molecular fossils—biomolecules, usually lipids, that resist decay and so preserve a chemical signature of ancient life.
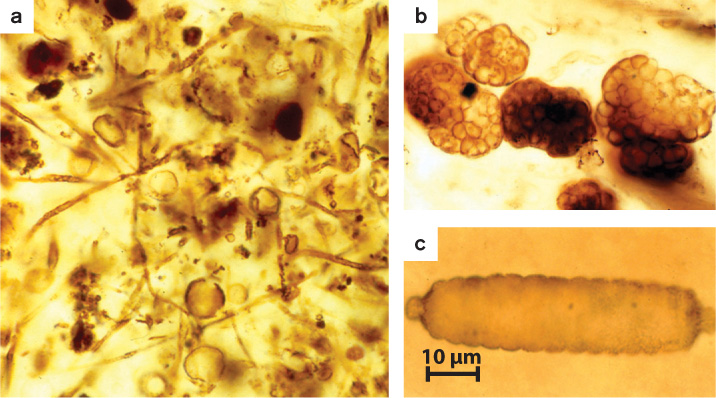

26.6.1 Life originated early in our planet’s history.
The Earth is about 4.5 billion years old, but few sedimentary rocks have survived destruction by geologic processes and remain to document our planet’s earliest history. Sedimentary rocks deposited nearly 3.5 billion years ago in Western Australia and South Africa provide some of our earliest records of Earth’s history. Remarkably, they preserve a scarce but discernible signature of life. Tiny organic structures preserved in these rocks have been interpreted as microfossils, although this conclusion remains controversial. More persuasive evidence of life in early oceans is provided by stromatolites. Stromatolites in ancient rocks are strikingly similar to modern structures formed by microbial communities, and so provide evidence of life that goes back about 3.5 billion years (Fig. 26.21). Also, because purely physical processes cannot explain the isotopic composition of carbon in very old limestones and organic matter, we can conclude that a biological carbon cycle existed 3.5 billion years ago (and, from the evidence of 3.8-billion-year-old rocks in Greenland, possibly earlier).
The chemistry of Earth’s oldest sedimentary rocks also shows that the early atmosphere and ocean contained little or no free oxygen. What would the carbon cycle look like on an oxygen-free planet? We know the answer from our discussion of prokaryotic metabolism. Photosynthesis can proceed in oxygen-poor waters, using H2S, H2, or Fe2+ as electron donors, and fermenters and anaerobic respirers can recycle carbon using electron acceptors such as SO42− and Fe3+. Geologic evidence suggests that iron played a particularly important role in cycling carbon for the first billion years of evolutionary history.
The evolution of cyanobacteria, with their capacity for oxygenic photosynthesis, made possible the accumulation of oxygen in the atmosphere and oceans. As discussed in Chapter 25, oxygen began to accumulate in the atmosphere about 2.4 billion years ago, making possible aerobic respiration and, eventually, our present-day carbon cycle. Both microfossils and molecular fossils indicate that cyanobacteria and other photosynthetic bacteria continued to dominate primary production until about 800 million years ago. Thus, our modern carbon cycle, powered overwhelmingly by oxygenic photosynthesis and with major participation by eukaryotic organisms, may have existed for only the last 20% of Earth’s history.
26.6.2 Prokaryotes have coevolved with eukaryotes.
Even after the rise of eukaryotic cells and, later, plants and animals, Bacteria and Archaea have remained essential to the functioning of biogeochemical cycles and hence to the maintenance of habitable environments on Earth. Prokaryotic organisms have also radiated into the many novel environments made possible by eukaryotes. We noted earlier that bacteria fix nitrogen in nodules formed on soybean roots. Soybeans and nitrogen-fixing bacteria evolved together, a linkage called coevolution (Chapter 47).
Coevolutionary relationships between prokaryotic microorganisms and eukaryotes are common in nature. As noted earlier, cows metabolize grass with the help of methanogenic archaeons in the rumen, a specialized digestive organ. The microbes enable the cow to gain nutrition from plant materials that could otherwise not be digested, and the benefit to the microorganisms is a steady supply of food. Similarly, clams that live near methane vents in the Gulf of Mexico have evolved specialized organs that house methane-oxidizing bacteria, providing them with a reliable source of nutrition.
Bacteria can affect animal behavior as well as nutrition. For example, some squid species attract mates by emitting light from specialized organs full of bioluminescent bacteria. The bacteria provide the squid with a mechanism for communication in the dark deep-sea environment, and the squid feeds the bacteria. Interestingly, many different kinds of bacteria live in the waters that surround the squid, but only the bioluminescent species accumulates in the squid’s light organ. The squid apparently sends molecular signals that attract just those bacteria.
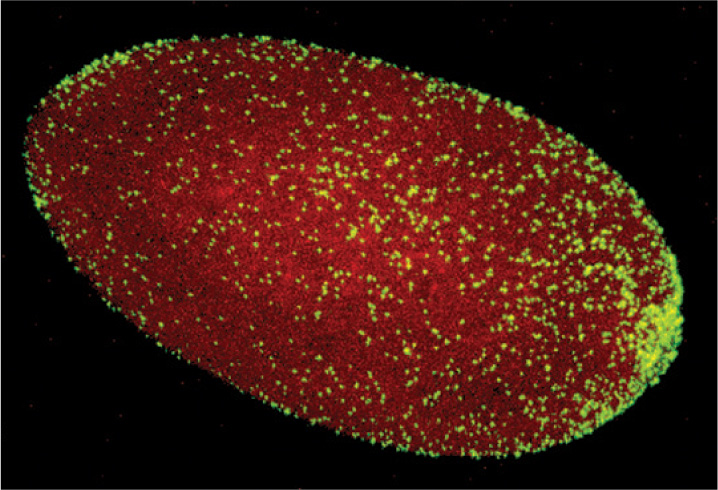
Bacteria can also influence animal reproduction. Earth supports more than a million species of insects, and perhaps two-thirds of these harbor the proteobacterial parasite Wolbachia within their tissues. A heterotroph that gains nutrition by metabolizing organic compounds supplied by its host, Wolbachia infects its host’s reproductive tissues and passes from one generation to the next in the host’s eggs (Fig. 26.22). Remarkably, Wolbachia strains ensure the integrity of their biological homes by manipulating the reproductive biology of the host. Many Wolbachia strains alter host reproductive cells so that the insect host can successfully reproduce only with partners infected by the same Wolbachia strain. Because Wolbachia cells are transferred by eggs and not sperm, they have little use for males. Consequently, some cause male host embryos to die, thus increasing the proportion of females. Others cause genetically male embryos to develop as females, or enable eggs to develop into female embryos without fertilization.
Wolbachia have another effect on their insect hosts that is highly relevant to human health: They commonly inhibit infection by viruses and the eukaryotic parasites that cause malaria. Recent research shows that Wolbachia populations in some fly species protect their hosts against infection by RNA viruses. Scientists introduced these bacteria into mosquitos that transmit dengue fever, a tropical disease that infects 50–100 million people each year. The Wolbachia-infected mosquitos were strongly resistant to the Dengue virus, which causes dengue fever. Moreover, when introduced into the wild, these virus-resistant forms rapidly expanded to dominate regional mosquito populations. Wolbachia parasites present a promising path to controlling dengue fever, malaria, and other insect-borne diseases.
All around us, plants, animals, and microorganisms live in intimate association—sometimes to our detriment, but commonly in mutually beneficial relationships that influence how we grow, develop, eat, and behave. Humans are no exception.
Case 5 The Human Microbiome: Diversity Within
26.6.3 How do intestinal bacteria influence human health?
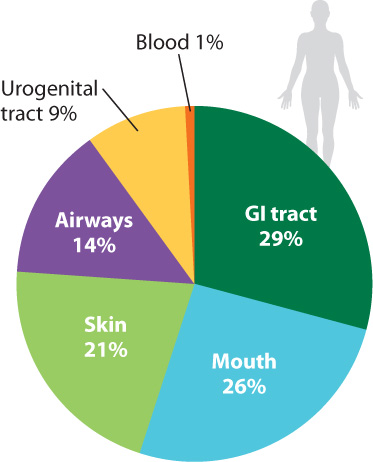
It has been estimated that the bacteria (and, to a much lesser extent, archaeons) in and on your body outnumber your own cells 10 to 1. Estimates of the number of microbial species that inhabit our bodies vary, but some 750 types have been identified in the mouth alone, and the list remains incomplete (Fig. 26.23). An equal number resides in the colon, and still more live on the skin and elsewhere. Some are transients, entering and leaving the body within a few bacterial generations. Others are specifically adapted for life within humans, forming complex communities of interacting species.
At present, much research is focused on the bacteria within our intestinal tracts. We commonly think of gut bacteria as harmful, but this perception arises from only a small—albeit devastating—subset of our microbial guests. Illnesses known to be caused by bacteria within our bodies include cholera, dysentery, and tuberculosis. Once the leading causes of death in New York and London, they remain major killers in Africa. Other diseases, not previously thought to be of microbial origin, are now known to be induced by bacteria—ulcers, for example, and stomach cancer, both mediated by the acid-tolerant bacterium Helicobacter pylori. More often than not, however, intestinal bacteria have a beneficial effect on our well-being. They help to break down food in our digestive system and secrete vitamins and other biomolecules into the colon for absorption into our tissues. Molecular signals from bacteria guide the proper development of cells that line the interior of our intestines.
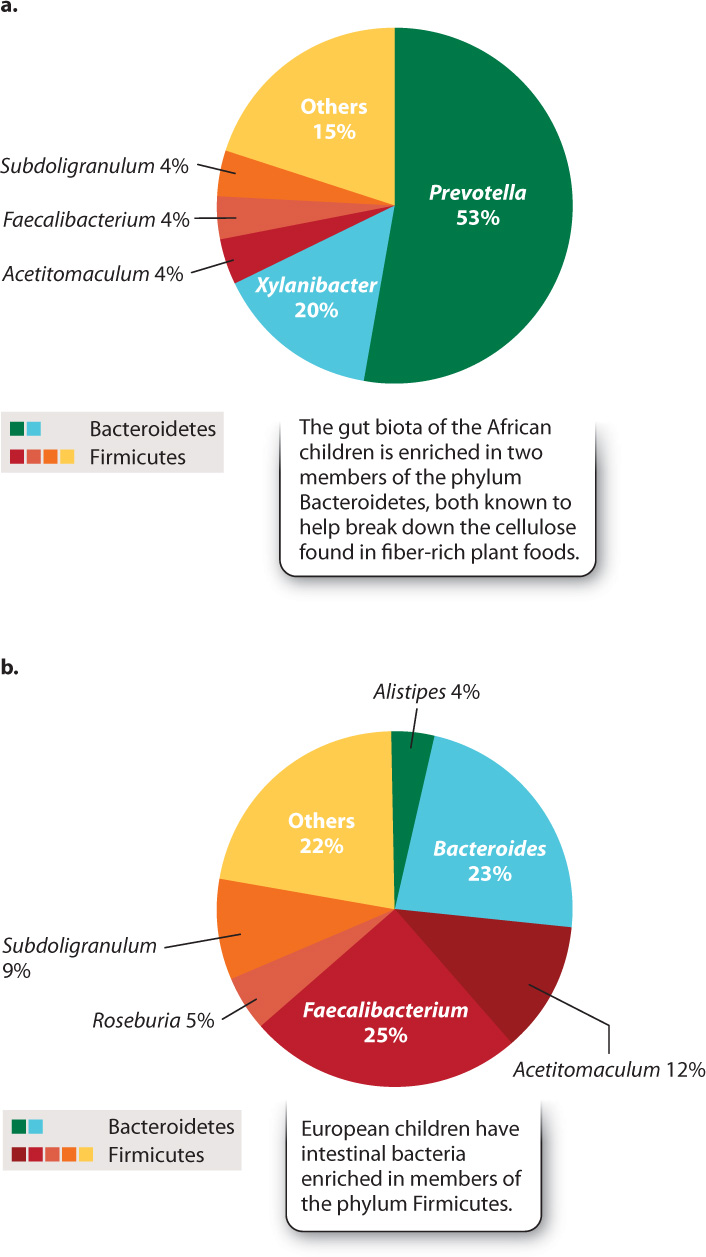
A fertilized human egg has no bacteria attached to it, so our microscopic passengers arrive by colonization—by infection, if you will. Research on children from Europe and rural Africa clearly shows the importance of diet in establishing our gut microbiota (Fig. 26.24). The African children, raised on a diet low in fat and animal protein but rich in plant matter, have gut microbiotas enriched in species of the phylum Bacteroidetes that are known to help digest cellulose. European children raised on a typical Western diet rich in sugar and animal fat lack these bacteria, harboring instead diverse members of the phylum Firmicutes. We do not understand the full ramifications of these differences, but active research suggests that they may help to explain the relative prominence in Western societies of allergies and other disorders involving the immune system.
We also influence our gut biota by ingesting antibiotics. Although prescribed for the control of pathogens, most antibiotics kill a wide range of bacteria and so can change the balance of our intestinal microbiota. Clinical studies show that the incidence of inflammatory bowel disease, a disabling inflammation of the colon, increases in humans who have been treated with antibiotics.
To understand the relationships between the human microbiome and human health, we need to know what constitutes a healthy gut biota, and this means studying the metabolism, ecology, and population genetics of the bacteria in our bodies. We need to know which bacteria have evolved to take advantage of the environments provided by the human digestive system, and which are “just passing through.” Future visits to the doctor may include routine genetic fingerprinting of our bacterial biota, as well as treatments designed to keep our microbiome—and, so, ourselves—healthy.