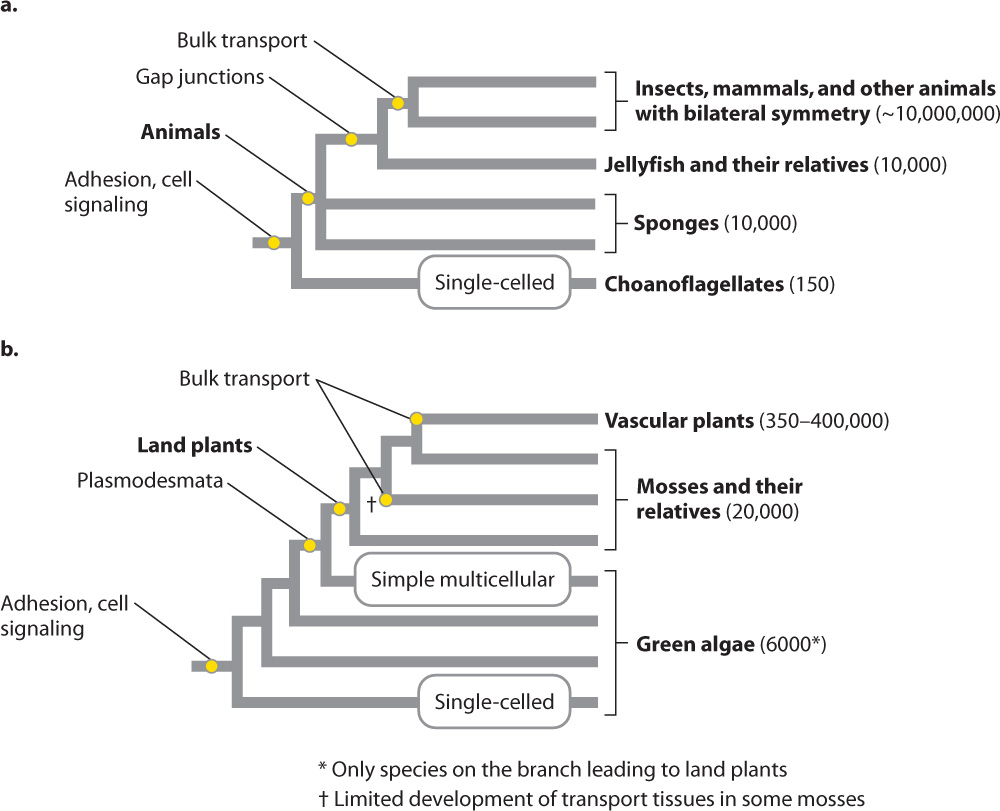
28.5 THE EVOLUTION OF COMPLEX MULTICELLULARITY
The gulf between an amoeba and a lobster seems vast, but research over the past decade increasingly shows how this apparent gap was bridged through a series of evolutionary innovations. Throughout this chapter, we have emphasized that complex multicellular organisms require mechanisms for cell adhesion, modes of communication between cells, and a genetic program to guide growth and development. Not only were all three required for the evolution of complex multicellular organisms, they also had to be acquired in a specific order. If the products of cell division don’t stick together in a pattern that has some usefulness in function, there can be no complex multicellularity. Adhesion, however, is not sufficient. It must be followed by mechanisms for communication between cells. Moreover, cells must be able to send molecular messages in a targeted or spatially specific pattern, facilitated in animals by gap junctions and in plants by plasmodesmata.
With these requirements in place, evolution would favor the increase and diversification of the genes that regulate growth and development, making possible more complex morphology and anatomy. The biological stage was finally set for the functional key to complex multicellularity: the differentiation of tissues and organs that govern the bulk transport of fluids, nutrients, signaling molecules, and oxygen through increasingly large and complex bodies. This differentiation freed organisms from the tight constraints imposed by diffusion. When all these features are placed onto phylogenies, they show both the predicted order of acquisition and the tremendous evolutionary consequences of complex multicellularity (Fig. 28.12).
28.5.1 Complex multicellularity appeared in the oceans 575 to 555 million years ago.
In Chapter 23, we noted that phylogenies based on living organisms make predictions about the fossil record. Characters and groups associated with lower branches in phylogenies should appear as earlier fossils than those associated with later branches. Although fossils don’t preserve molecular features, the record supports the phylogenetic pattern shown in Fig. 28.12.
Single-celled protists are found in sedimentary rocks as old as 1800 million years, and a number of simple multicellular forms have been discovered that are nearly as old (see Fig. 27.23). Nonetheless, the oldest fossils that record complex multicellularity occur in rocks that were deposited only 575 to 555 million years ago. Fig. 28.13a shows one of the oldest known animal fossils, a frondlike form preserved in 575-million-year-old rocks from Newfoundland, near the eastern tip of Canada. This fossil and many others found in rocks of this age are enigmatic. There is complex morphology to be sure, but it is difficult to understand these forms by comparing them to the body plans of living animals. The Newfoundland fossils show no evidence of head or tail, no limbs, and no opening that might have functioned as a mouth. They appear to be very simple organisms that gained both carbon and oxygen by diffusion. These fossils are long and wide, but they are not thick. In fact, it is likely that active metabolism occurred only along the surfaces of these structures.

Such animals lie near the base of the animal tree (Chapter 44), and probably would have developed under the guidance of regulatory genes similar to those present in modern sponges or, perhaps, jellyfish. By 560–555 million years ago, more complex animals, with distinct head and tail, top and bottom, left and right enter the record (Fig. 28.13b). At the same time, we begin to see tracks and trails made by animals whose muscles enabled them to move across and through surface sediments (Fig. 28.13c), an indirect but complementary record of increasing animal complexity. These, then, are the first known occurrences of the types of animal that dominate ecosystems today, both in the sea and on land. These animals must have possessed a diverse array of genes to guide development, something akin to those seen today in insects or snails.
Clearly, by 575–555 million years ago, the animal tree was beginning to branch. This, however, raises a new question: Why did complex multicellularity appear so much later than simple multicellular organisms?
28.5.2 Oxygen is necessary for complex multicellular life.
Earlier in the chapter, we discussed the key biological requirements for complex multicellularity. There is a critical environmental requirement as well: the presence of oxygen. Only the oxidation of organic molecules by O2 provides sufficient energy to support biological communities that include large and active predators like wolves and lions. Not only do other potential electron acceptors for respiration, like sulfate and ferric iron, occur in much lower abundances, they are not gases and so do not accumulate in air. And only oxygen that is present in concentrations approaching those of the present day is able to diffuse into the interior cells of large, active organisms. On our planet—and probably, on all planets—no other oxidant is both sufficiently abundant and has the oxidizing power needed to support the biology of large complex organisms.
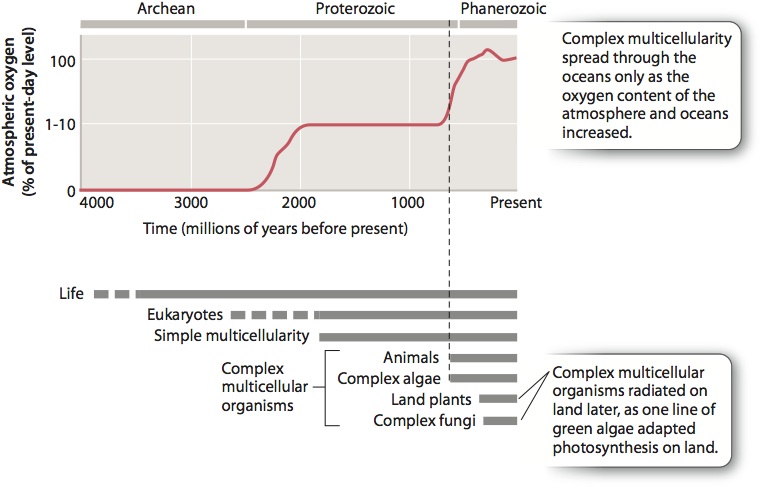
On the present-day Earth, lake or ocean bottoms with oxygen levels below about 10% of surface levels support few animals, and those that do tend to be tiny. Large, active, and diverse animals can live only in oxygen-rich environments. The clear restrictions on animal form and diversity observed along an environmental gradient of oxygen availability have important implications for the distribution of large, active, and diverse animals through time. We noted in Chapter 26 that oxygen first began to accumulate in the atmosphere and surface oceans about 2400 million years ago, but the chemistry of sedimentary rocks deposited after that time tells us that oxygen levels remained low for a very long time. Our modern world of abundant oxygen came to exist only 580–560 million years ago, about the time when fossils first record complex multicellular organisms (Fig. 28.14).
Scientists continue to debate the causes of this environmental transformation, but the correspondence in time between oxygen enrichment and the first appearance of complex animals (and algae) suggests that more oxygen permitted greater size. Greater size in turn created opportunities for tissue differentiation, leading to the bulk transport of nutrients and signaling molecules. The evolution of bulk transport in turn permitted still larger size, setting up a positive feedback that eventually resulted in the complex marine organisms we see today.
With morphological complexity came new functions, including predation on other animals. Protozoan predators capture other microorganisms, but do so one cell at a time. In contrast, animals that obtain food by filtering seawater gather cells by the thousands, and larger animals can eat smaller ones, opening up endless possibilities for evolutionary specialization and, therefore, diversification. Among photosynthetic organisms, multicellular red and green algae were able to establish populations in wave-swept coastlines and other environments where simpler organisms lacked the mechanical strength to hold their position along the shoreline.
The key point is that complex multicellular organisms didn’t succeed by doing the same things as simpler eukaryotes. In an oxygen-rich world, complex multicellularity spread through the oceans because it opened up new and unprecedented evolutionary possibilities.
28.5.3 Land plants evolved from green algae that could carry out photosynthesis on land.
Complex multicellular land plants originated about 460 million years ago, well after marine animals and algae. In some ways, the pattern of evolution of land plants parallels that of animals. Ancestral green algae evolved molecular means for cell–cell adhesion and communication between cells, and the algae that form the sister group to land plants share with them the innovation of plasmodesmata. Then, as they developed larger and more complex three-dimensional bodies, plant ancestors evolved a succession of genes and their protein products to guide cell division, expansion, and differentiation. Land plants, however, had a different challenge from that of animals. The diversification of plants across the land surface requires that photosynthesis, evolved earlier by aquatic organisms, be carried out within tissues bathed by air. It also requires that nutrients and water be absorbed from the soil and transported throughout the plants rather than simply taken in by diffusion from the surrounding water.
Fossils indicate that by 400 million years ago, plants with specialized tissues for bulk transport of water and nutrients had begun to spread across the continents. As plant biomass built up on land, it created opportunities for additional types of complex multicellular organism. In particular, the fungi, which decompose organic matter, radiated by taking advantage of this resource (Fig. 28.15), evolving complex multicellularity in two distinct lineages (Chapter 34).

28.5.4 Regulatory genes played an important role in the evolution of complex multicellular organisms.
Complex multicellular organisms account for a large proportion of all eukaryotic species, and the evolutionary consequences of complex multicellularity are seen clearly when we compare the diversity of complex multicellular groups with their simpler phylogenetic sisters. Choanoflagellates, for example, number about 150 species, simple animals such as sponges and jellyfish about 20,000, and complex animals with circulatory systems that circumvent diffusion perhaps as many as 10 million. Plants and their relatives show a comparable pattern. There are about 6000 species of green algae on the branch that includes land plants, but about 400,000 species of anatomically complex land plants capable of bulk transport. Similarly, complex fungi and red algae include more species by an order of magnitude than their simpler relatives.
How did this immense diversity arise? At one level, the answer is functional and ecological. Complex multicellular organisms can perform a range of functions that simpler organisms cannot, and so they have evolved many specific types of interaction with other organisms and the physical environment. This explanation, however, prompts another question: What is the genetic basis for the bewildering range of sizes and shapes displayed by complex multicellular organisms?
The answer has to do with the network of genes that guide development. Since the 1950s, biologists have learned a remarkable amount about this genetic network and its host of interacting genes. Many of the genes involved in development are what we might consider middle managers: A molecular signal induces the expression of a gene, and the protein product, in turn, prompts the expression or repression of another gene. It is the complex interplay of these genetic switches—turning specific genes on in one cell and off in another, depending on where those cells occur in the developing body—that results in crabs with large pincers or butterflies with patterned wings (Fig. 28.16).
FIG. 28.16What controls color pattern in butterfly wings?
BACKGROUND Butterfly wings commonly show a striking pattern of color, including circular features known as eyespots. Eyespots have adaptive value, for example in deterring predation by birds.
HYPOTHESIS The expression of regulatory genes during wing development governs eyespot formation on wing surfaces.
EXPERIMENT Paul Brakefield and his colleagues mapped the expression of a regulatory gene called Distalless in developing butterfly wings. Distalless genes were fused to genes for green fluorescent protein (GFP), a protein that glows vivid green when illuminated under blue light. GFP would then be expressed along with Distalless, allowing the spatial pattern of Distalless expression in developing wings to be followed by GFP visualization.
RESULTS The expression pattern of Distalless in developing wings closely resembles the pattern of eyespots on the wing. Moreover, mutations that change the spatial pattern of Distalless expression result in different eyespot patterns.
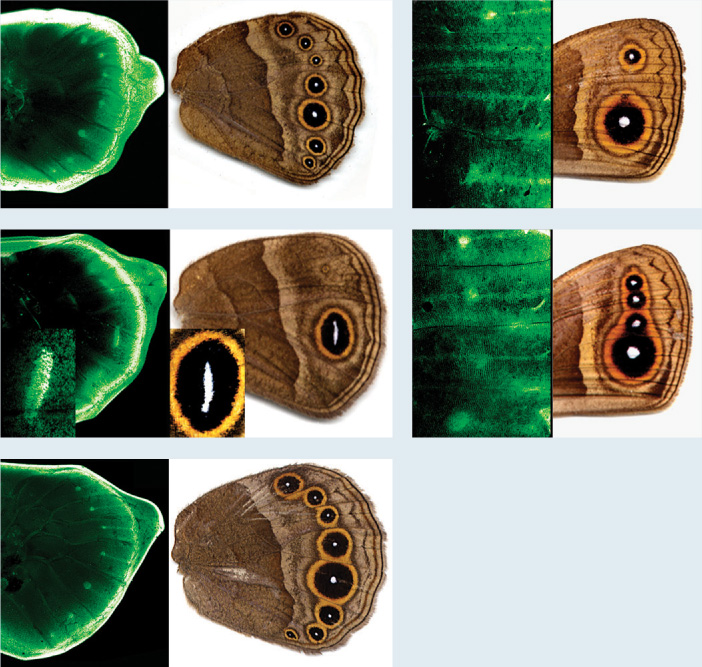
The five pairs show the pattern of Distalless gene expression and eyespot development in wild-type (top left) and mutant butterfly wings. High levels of Distalless expression (green dots and ovals) define the centers of eyespots.
CONCLUSION Regulatory genes play an important role in butterfly wing coloration, and mutations in these genes can account for differences in wing coloration among species.
SOURCE Brakefield, P. M., J. Gates, D. Keys, F. Kesbeke, P. J. Wijngaarden, A. Monteiro, V. French, and S. B. Carroll. 1996. “Development, Plasticity and Evolution of Butterfly Eyespot Patterns.” Nature 384:236–242.
It makes sense that if regulatory genes guide development of the body in each complex multicellular species, then mutations in regulatory genes may account for many of the differences we observe among different species. Our growing understanding of how developmental genes underpin evolutionary change has given rise to a whole new field of biology called evolutionary-developmental biology, or evo-devo for short.
In evo-devo research, scientists compare the genetic programs for growth and development in species found on different branches of phylogenetic trees. In addition to wanting to discover the molecular mechanisms that guide development in individual species, these scientists aim to understand the genetic differences associated with differences in form and function among species. Evo-devo is an exciting and rapidly expanding field of research because it is helping to illuminate the long-suspected relationship between the development of individuals and patterns of evolutionary relatedness among species. Continuing research promises to shed new light on the similarities and differences among plants, animals, and other complex multicellular organisms.