29.3 THE STEM: TRANSPORT OF WATER THROUGH XYLEM
On a summer day, a tree can transport many hundreds of liters of water from the soil to its leaves. This is an impressive feat given that it is accomplished without any moving parts. Even more remarkable, trees and other plants transport water without any direct expenditure of energy. The upward transport of water is possible because the structure of vascular plants allows them to use the evaporation of water from leaves to pull water from the soil.
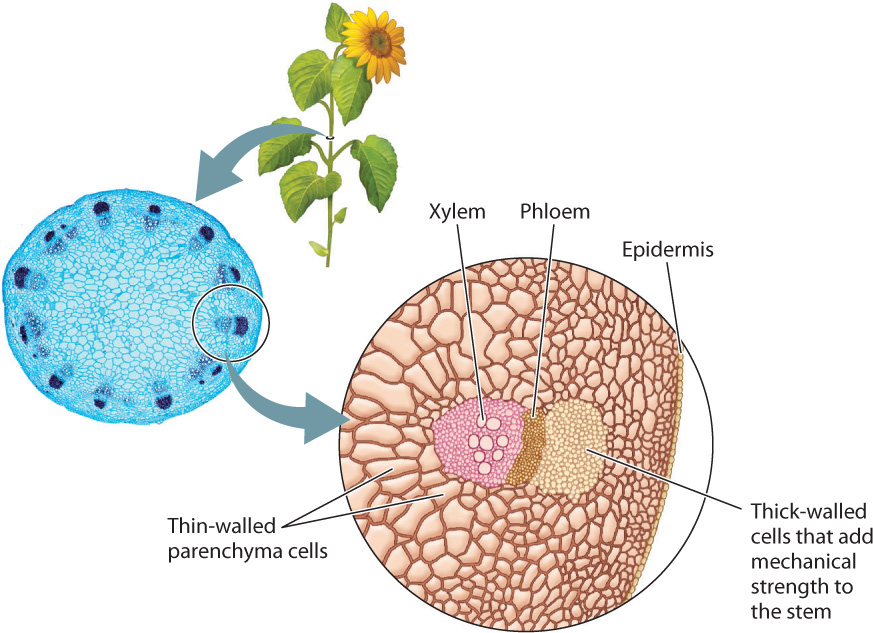
Fig. 29.10 shows the cross section of a sunflower stem. Like a leaf, it has a surface layer of epidermal cells. This layer encloses thin-walled parenchyma cells in the interior. Notice that the stem also contains differentiated tissues that lie in a ring near the outside of the stem. These are the vascular tissues, which form a continuous pathway that extends from near the tips of the roots, through the stem, and into the network of veins within leaves. The outer tissue, called phloem, transports carbohydrates from leaves to the rest of the plant body. The inner tissue, called xylem, transports water and nutrients from the roots to the leaves.
29.3.1 Xylem provides a low-resistance pathway for the movement of water.
Water travels with relative ease through xylem because of the structure of the water-transporting cells within this tissue. As they develop, these cells become greatly elongated. When they complete their growth, they secrete an additional wall layer that is very thick and which contains lignin, a chemical compound that increases mechanical strength. Most remarkable is the final stage of development, when the nucleus and cytoplasm are lost, leaving behind only the cell walls.
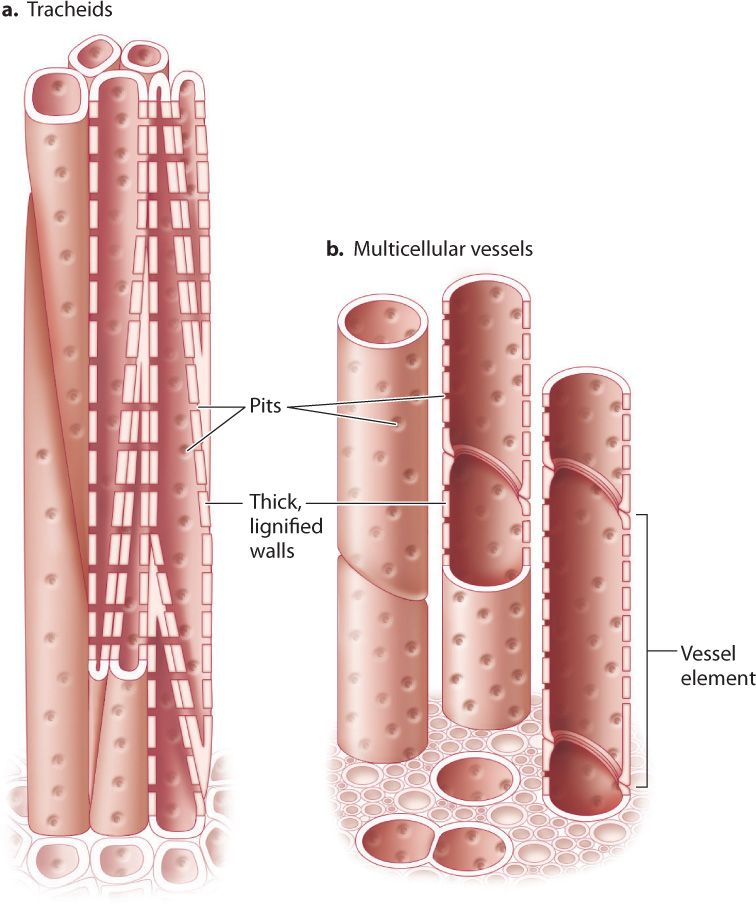
These thick walls form a hollow conduit through which water can flow (Fig. 29.11). Water enters and exits xylem conduits through pits, circular or ovoid regions in the walls where the lignified cell wall layer is not produced. Instead, pits contain only the thin, water-permeable cell wall that surrounded each cell as it grew. As we will see, pits play an important role in xylem because they allow the passage of water, but not air, from one conduit to another.
Xylem conduits can be formed from a single cell or multiple cells stacked to form a hollow tube. Unicellular conduits are called tracheids (Fig. 29.11a), and multicellular conduits are called vessels (Fig. 29.11b). Because tracheids are the product of a single cell, they are typically 4 to 40 μm in diameter and no more than 2 to 3 cm long. Vessels, which are made up of individual cells called vessel elements, can be much wider and longer. Vessel diameters range from 5 to 500 μm, and lengths can be up to several meters.
Water enters a tracheid through pits, travels upward through the conduit interior, and then flows outward through other pits into an adjacent, partially overlapping, tracheid. Water also enters and exits a vessel through pits. In contrast to tracheids, however, once the water is inside the vessel, little or nothing blocks the flow of water from one cell to the next. That is because during the development of a xylem vessel, the end walls of the vessel elements are digested away, allowing water to flow along the entire length of the vessel without having to cross any pits. At the end of a vessel, however, the water must flow through pits if it is to enter an adjacent vessel and thereby continue its journey from the soil to the leaves.
The rate at which water moves through xylem is a function of both the number of conduits and their size. Conduit length determines how often water must flow across pits, which exert a significant resistance to flow. Conduit width also has a strong effect on the rate of water transport. Like the flow through pipes, water flow through xylem conduits is greater when the conduits are wider. The flow is proportional to the radius of the conduit raised to the fourth power, so doubling the radius increases flow sixteenfold. Because vessels are both longer and wider than tracheids, plants with vessels achieve greater rates of water transport than is possible through tracheids alone. Tracheids are the most common conduits in lycophytes, ferns and horsetails, and gymnosperms, whereas vessels are the principal conduit in angiosperms.
29.3.2 Water is pulled through xylem by an evaporative pump.
If you cut a plant’s roots off under water, the leaves continue to transpire for some time. The persistence of transpiration when roots are absent demonstrates that the driving force for water transport is not generated in the roots, but instead comes from the leaves. In essence, water is pulled through xylem from above rather than being pushed from below.
The forces pulling water upward through the plant are large. Not only must these forces be able to lift water against gravity, they must also be able to pull water from the soil, which becomes increasingly difficult as the soil dries. In addition, they must be able to overcome the physical resistance associated with moving water through the plant itself. To replace water lost by transpiration with water pulled from the soil, the leaves must exert forces that are many times greater than the suctions that we can generate with a vacuum pump (Fig. 29.12). How do leaves exert this force?
FIG. 29.12How large are the forces that allow leaves to pull water from the soil?
BACKGROUND The idea that water is pulled through the plant by forces generated in leaves was first suggested in 1895. However, without a way to measure these forces, there was no way to know how large they were.
HYPOTHESIS In 1912, German physiologist Otto Renner hypothesized that leaves are able to exert stronger suctions than could be generated with a vacuum pump.
EXPERIMENT Renner measured the rate at which water flowed from a reservoir into the cut tip of a branch of a transpiring plant. He next cut off about 10 cm of the branch and attached a vacuum pump in place of the transpiring plant. He then compared the flow rate through the branch when attached to the transpiring plant with the flow rate generated by the vacuum pump.
RESULTS Renner found that the flow rates generated by transpiring plants were two to nine times greater than the flow rates generated by the vacuum pump.
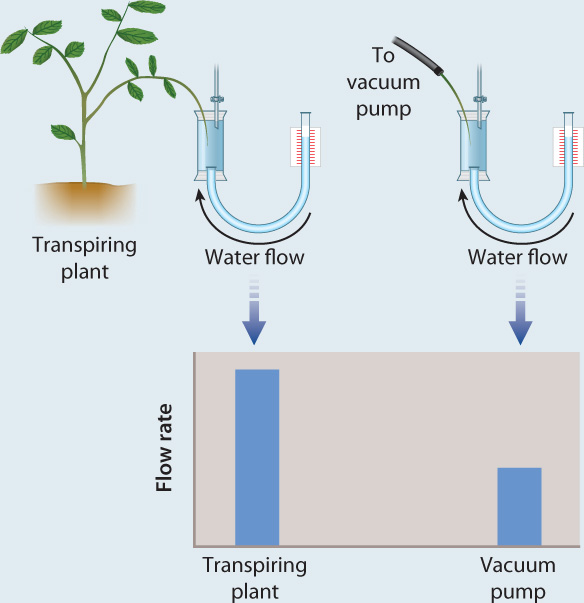
CONCLUSION A transpiring plant pulls water through a branch faster than a vacuum pump. Therefore, the pulling force generated by a transpiring plant must be greater than that of a vacuum pump. Because vacuum pumps create suctions by reducing the pressure in the air, the maximum suction that a vacuum pump can generate is 1 atmosphere. Thus, the maximum height that one can lift water using a vacuum pump is 10 m (33 feet). The forces that pull water through plants have no comparable limit because they are generated within the hydrated cell wall. This is why plants can pull water from dry soils and why they can grow to more than 100 m in height.
FOLLOW-UP WORK In 1965, Per Fredrick Scholander developed a pressurization technique that uses reverse osmosis to measure the magnitude of the suctions exerted by leaves of intact plants. He used this technique to show that the leaves at the top of a Douglas fir tree 100 m tall exert greater pulling forces than do leaves closer to the ground.
SOURCES Renner, O. 1925. “Zum Nachweis negativer Drucke im Gefässwasser bewurzeler Holzgewachse.” Flora 118/119:402-408.; Scholander, P. F., H. T. Hammel, E. D. Bradstreet, and E. A. Hemmingson. 1965. “Sap Pressure in Vascular Plants.” Science 148:339–346.
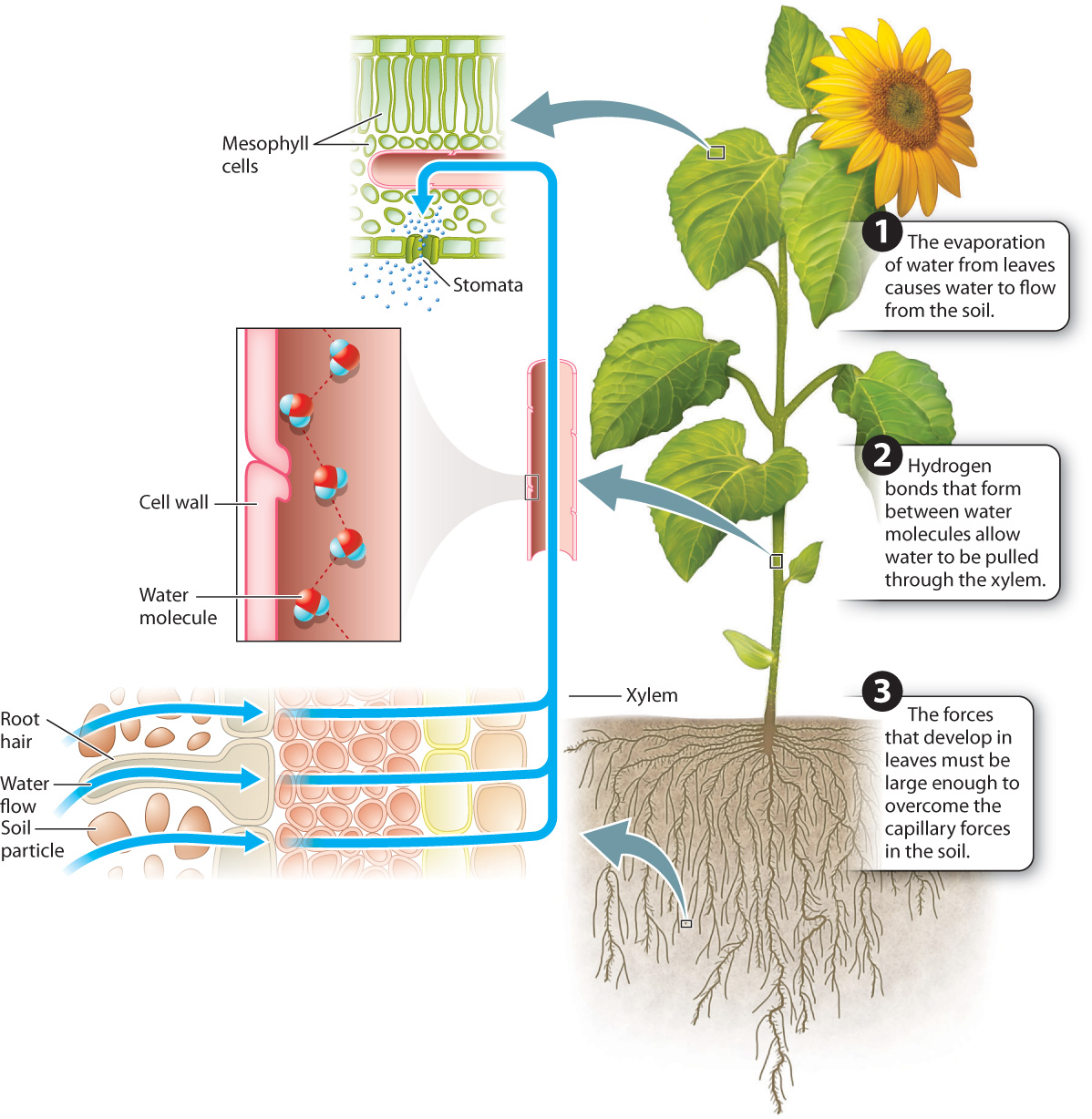
When stomata are open, water evaporates from the walls of cells lining the intercellular air spaces of leaves. The partial dehydration of the cell walls creates a force that pulls water towards the sites of evaporation. One hypothesis for how this force is generated is that water is pulled by capillary action into spaces between the cellulose microfibrils in the cell wall, the same reason that water is drawn into a sponge. A second hypothesis is that the pectin gel in which the cellulose microfibrils are embedded causes water to flow into the partially dehydrated cell walls by osmosis. Osmotic forces can be generated in cell walls because the negatively charged pectin network restricts the diffusion of positively charged ions, much as the plasma membrane maintains a high concentration of solutes in the cytoplasm.
Once generated by the evaporation of water from leaves, this force is transmitted through the xylem, beginning in the leaf veins, then down through the stem, and out through the roots to the soil (Fig. 29.13). Water can be pulled through xylem because of the strong hydrogen bonds that form between water molecules. This mechanism of water transport only works, however, if there is a continuous column of water in the xylem that extends from the roots to the leaves.
29.3.3 The structure of xylem conduits reduces the risks of collapse and cavitation.
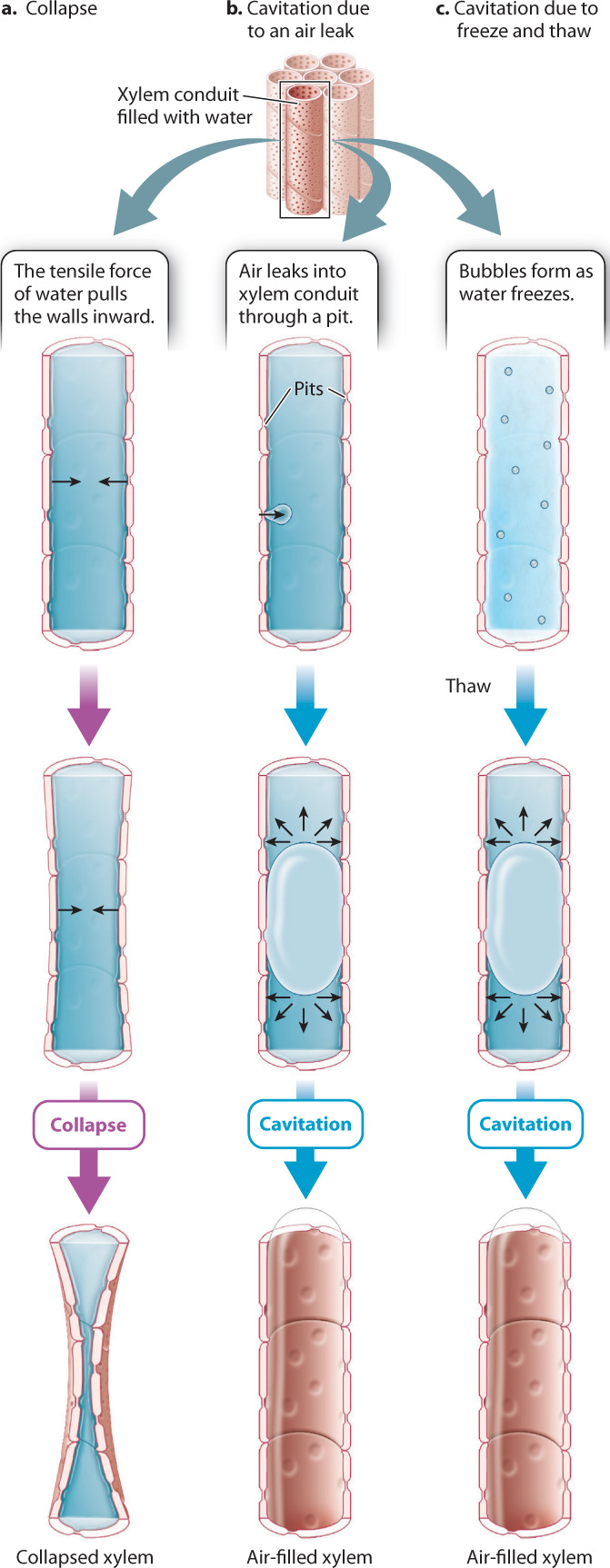
The fact that water is pulled from the soil means that xylem conduits must be structured in such a way as to minimize two distinct risks (Fig. 29.14). The first is the danger of collapse (Fig. 29.14a). If you suck too hard on a drinking straw, it will collapse inward, blocking flow. Much the same thing can happen in xylem. Although in metabolic terms, lignin is more costly to produce than cellulose, lignin makes conduit walls rigid, reducing the risk of collapse.
The second danger associated with pulling water through the xylem is cavitation, which occurs when the water in a conduit is abruptly replaced by water vapor. Because cavitation disrupts the continuity of the water column, cavitated conduits can no longer transport water from the soil. Cavitation in xylem results from the presence of microscopic gas bubbles in the water that are sufficiently large that they expand under the tensile, or pulling, force exerted by the leaves. The microscopic bubbles that cause cavitation can be formed in either of two ways. The first is if an air bubble is pulled through a pit because of lower pressure in the water compared to the air (Fig. 29.14b). The larger the tensile forces exerted by leaves, the more likely it is that air will be pulled across a pit. Thus, higher rates of transpiration increase the risk of cavitation.
The second way that gas bubbles can form within xylem is if gases come out of solution during freezing (Fig. 29.14c). Gases are much less soluble in ice than in water, so as a conduit freezes, dissolved gas molecules aggregate into tiny bubbles that can cause cavitation when the conduit thaws. Wide vessels are especially vulnerable to cavitation at freezing temperatures. The susceptibility of wide vessels to cavitation partly explains why boreal (that is, subarctic) forests contain few angiosperms, which commonly have large vessel diameters.
Once cavitation has occurred, the liquid phase will not re-form as long as tension persists within the xylem. Thus, xylem is organized in ways that minimize the likelihood and impact of cavitation. For example, xylem consists of many conduits in parallel, so the loss of any one conduit to cavitation does not result in a major loss of transport capacity. Similarly, as water flows from the soil to the leaves, it passes from one conduit to another, each one of finite length. The likelihood that cavitation will spread is thereby reduced because air can be pulled through pits only when the tensile forces in the xylem are large.
Quick Check 3
How is the statement that water is transported through the xylem without requiring any input in energy by the plant both correct and incorrect?