30.1 THE PLANT LIFE CYCLE AND EVOLUTION OF POLLEN AND SEEDS
Plants did not evolve the ability to reproduce on land all at once. Early diverging lineages evolved the capacity to disperse their offspring through the air, but they still required water for fertilization. How could these plants exist in both water and air? They couldn’t—at least not at the same time. Instead, early land plants evolved a life cycle in which one generation, or phase of the life cycle, released sperm into a moist environment and the following generation dispersed offspring through the air. It was only with the evolution of pollen and seeds that plants no longer depended on external sources of water for fertilization. Yet the pattern of alternating generations remains a key feature of the plant life cycle.
30.1.1 The algal sister groups of land plants have one multicellular generation in their life cycle.
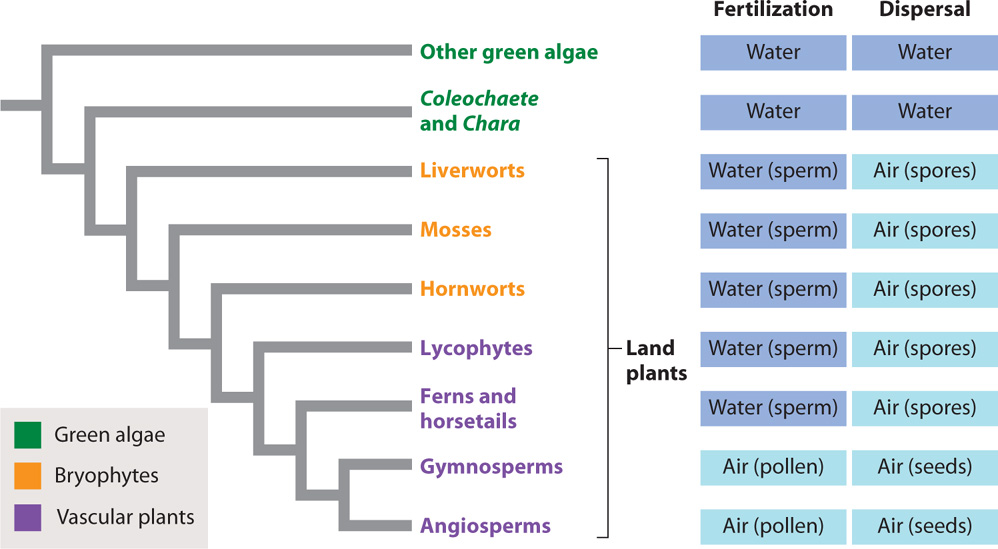
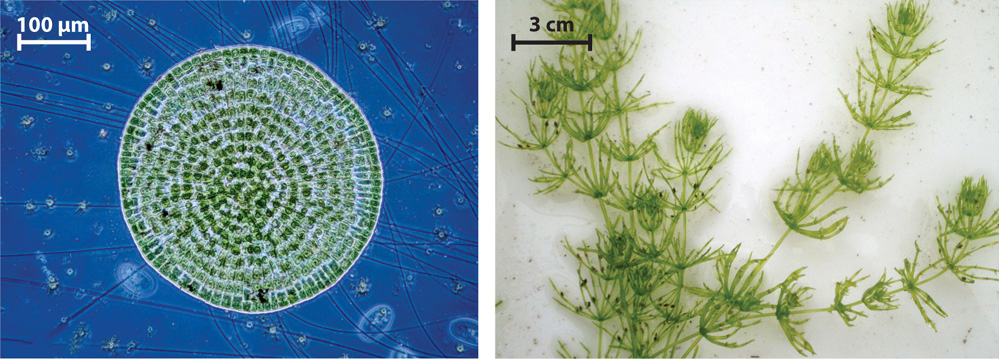
Molecular sequence comparisons show that two groups of green algae are most closely related to land plants (Fig. 30.1): Coleochaete, an inconspicuous green pincushion that grows on aquatic plants or rocks in freshwater environments, and the erect stonewort Chara (and closely related genera) found along the margins of lakes and estuaries (Fig. 30.2). Like all sexually reproducing eukaryotes (Chapter 27), these algae alternate between a diploid (2n) phase and a haploid (1n) phase. The diploid phase is generated by the fusion of haploid gametes to form a zygote, and the haploid phase is generated from diploid cells by meiosis.
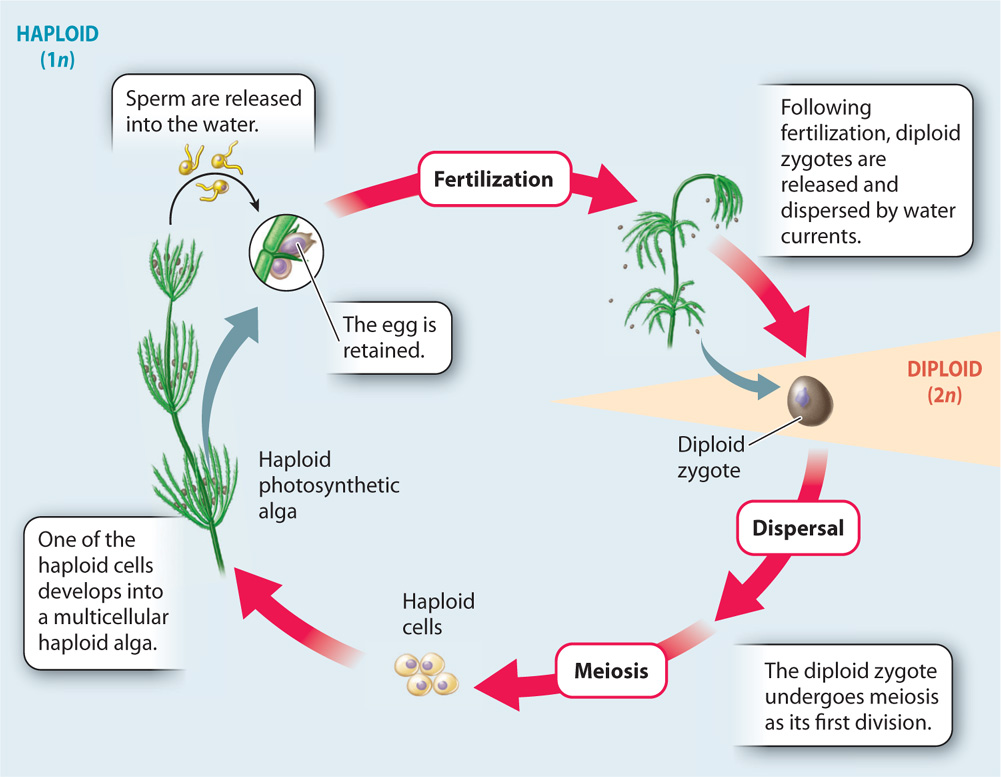
In animals, the multicellular body consists of diploid cells. Coleochaete and Chara have a multicellular body as well, shown in Fig. 30.2, but it consists entirely of haploid cells. Specialized cells of the haploid body produce haploid eggs and sperm by mitosis within multicellular reproductive organs. The egg is retained within the female reproductive organ and the sperm are released into the water. Fertilization results when one egg and one sperm fuse to form a diploid zygote. The zygote then undergoes meiosis to produce haploid cells that give rise to a new multicellular haploid generation (Fig. 30.3). In Coleochaete, the haploid products of meiosis disperse by swimming through the water, propelled by flagella. In Chara, the zygotes disperse, carried by water currents until they settle and undergo meiosis.
In Chara and Coleochaete, both fertilization and dispersal take place in water. Thus, these algae are as dependent on water as fish are. Living along the margins of lakes and streams, however, Chara and Coleochaete are occasionally left high and dry by drought. Their photosynthetic bodies shrivel quickly, but these algae survive because their zygotes form a protective wall that allows the cell inside to tolerate exposure to air. When water once again covers the resting zygotes, they undergo meiosis and the resulting haploid cells escape from their protective coat. This is the condition from which the more complicated life cycles of land plants evolved.
30.1.2 Bryophytes illustrate how the alternation of generations allows the dispersal of spores in the air.
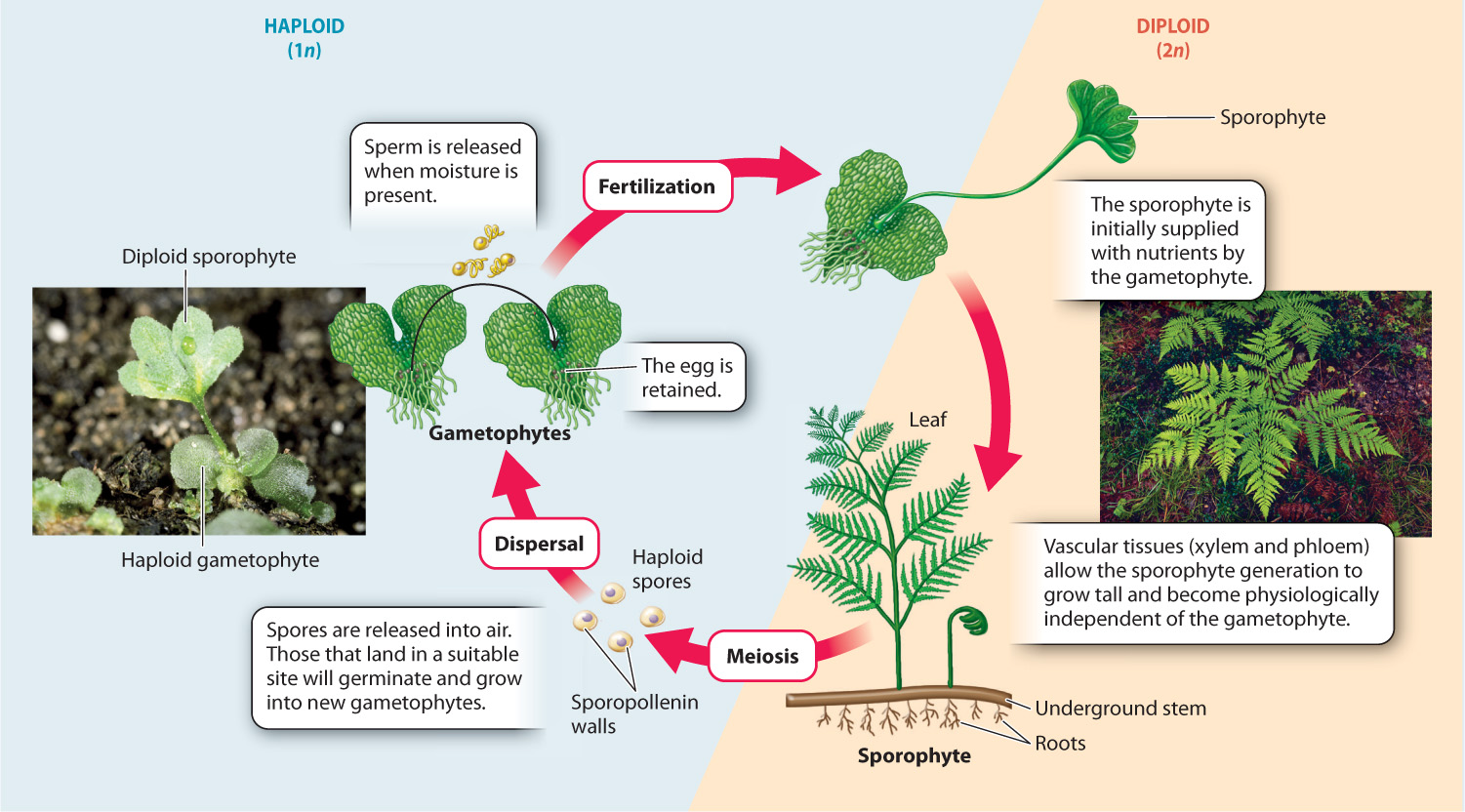
As plants moved onto land, their life cycle pulled them in two directions. Fertilization requires that plants remain near the ground, where water is most likely to be found, but dispersal in air requires that they grow tall, away from that water. In Chapter 29, we saw that the bryophytes—mosses, hornworts, and liverworts—make up the earliest branches on the phylogenetic tree of land plants (see Fig. 30.1). Mosses, hornworts, and liverworts share many reproductive traits. Here, we use Polytrichum commune, a moss common in forests and the edges of fields, to illustrate how movement onto land was accompanied by the evolution of two multicellular generations: one specialized for fertilization and the other for dispersal.
Like Chara and Coleochaete, Polytrichum has a photosynthetic body—the familiar green tufts observed on the forest floor—that is made of haploid cells and that forms gametes by mitotic division of specialized cells. Sperm travel to eggs retained within reproductive organs, and the fusion of egg and sperm gives rise to a diploid zygote.
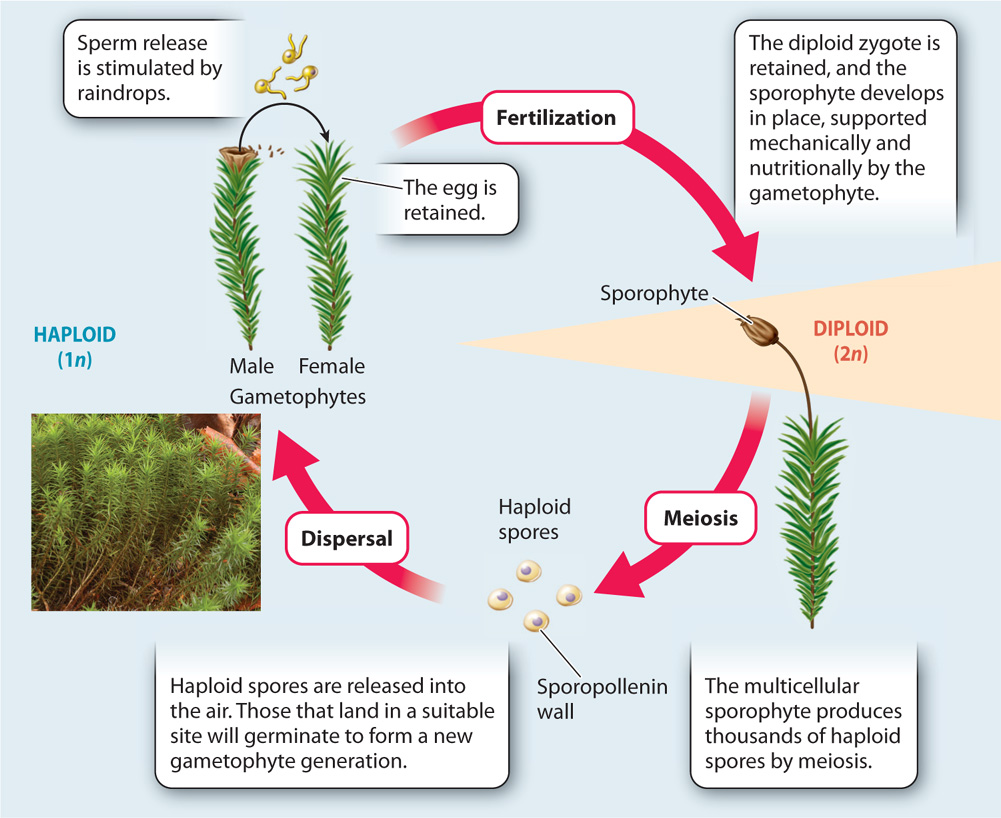
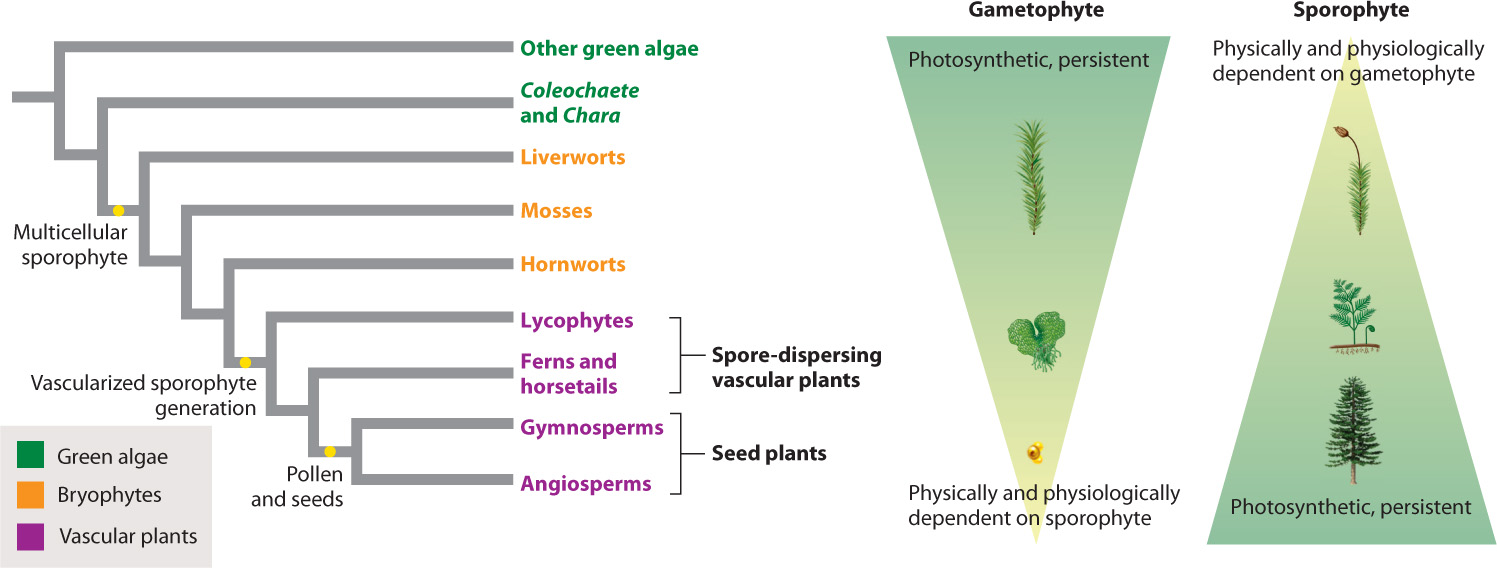
At this point, the life cycles of the green algae and Polytrichum diverge. In the moss, the zygote does not undergo meiosis and it does not disperse. Instead, the zygote is retained within the female reproductive organ, where it divides repeatedly by mitosis to produce a new multicellular generation—this one made of diploid cells (Fig. 30.4). Some cells within this diploid body eventually undergo meiosis, giving rise to haploid spores, cells that disperse and give rise to a new haploid generation. Because the diploid multicellular generation gives rise to spores, it is called the sporophyte, and because the haploid multicellular generation gives rise to gametes, it is called the gametophyte. The resulting life cycle, in which a haploid gametophyte and a diploid sporophyte follow one after the other, is called alternation of generations, and it describes the basic life cycle of all land plants.
Because land plants are descended from green algae with only one haploid generation, we can infer that the diploid sporophyte of land plants is an evolutionary novelty on this branch of the phylogenetic tree. How might this new generation have made it possible for plants to colonize land?
To answer this question, we need to identify the sporophyte of Polytrichum. Late in the growing season, the green tufts of this moss sprout an extension—a small brown capsule at the end of a cylindrical stalk a few centimeters high (Fig. 30.5a). This is the sporophyte, which originated from a fertilized egg. Because the fertilized egg was retained within the female reproductive organ, the sporophyte grows directly out of the gametophyte’s body. Thus, the Polytrichum specimen pictured in Fig. 30.5a shows both the gametophyte (green) and the sporophyte (brown). The gametophyte is photosynthetically self-sufficient. In contrast, the sporophyte obtains water and nutrients needed for its growth from the gametophyte.

The multicellular sporophyte enhances the ability of plants to disperse on land. The capsule at the top of the Polytrichum sporophyte is a sporangium, a structure in which many thousands of cells undergo meiosis, producing large numbers of haploid spores. Spores are ideally suited for transport through the air because, being small, they can be carried for thousands of kilometers by the wind. But at the same time, their tiny size puts spores at risk of being washed from the air by raindrops. The sporangia of many bryophytes release their spores only when the air is dry. In Polytrichum, the sporangium has small openings from which it releases spores the way a salt shaker releases salt. More common is the presence of a ring of teethlike projections that curl over the top of the sporangia. These bend backward when air humidity is low, flinging the spores into the air (Fig. 30.5b).
What protects the spores from drying out or from exposure to damaging ultraviolet radiation as they travel through the air? Earlier, we noted that the zygotes of Chara and Coleochaete secrete a wall that protects the zygote within from desiccation. The wall contains sporopollenin, a complex mixture of polymers that is remarkably resistant to environmental stresses such as ultraviolet radiation and desiccation. In mosses and other land plants, it is the spore, not the zygote, that secretes sporopollenin. Thus, early land plants had a capacity present in their algal ancestors—the ability to synthesize sporopollenin—but the timing of gene expression changed in these plants. Coated in sporopollenin, the spores cannot swim like meiotic products of Coleochaete. Instead, they are dispersed by wind to new locations, where they germinate to form a new haploid generation. Thus, the evolution of the multicellular sporophyte generation permitted land plants to make many spores and disperse them across large distances on land.
What about fertilization, the other point in the life cycle where Chara and Coleochaete require water? Like their algal ancestors, Polytrichum and other bryophytes rely on water to transport sperm to egg. Sperm released into the environment swim through thin films of water in soil and on the surface of the egg-bearing gametophyte. In addition, bryophytes tend to be small, so the distance between male and female gametes does not exceed the distance over which the sperm can swim, and they produce their gametes near the ground, where the likelihood of encountering a continuous film of water is greatest. A reliance on external water for fertilization restricts the growth of bryophytes to habitats that are, at least intermittently, wet. Many bryophytes release their sperm only when agitated by raindrops. Raindrops signal the presence of surface water, and their impact can splash sperm farther than they could swim on their own.
Quick Check 1
Give two reasons why spores are better suited for dispersal on land than are sperm.
30.1.3 Vascular plants evolved a large photosynthetic sporophyte generation.
The first two lineages of vascular plants—the lycophytes and the ferns and horsetails—depend on swimming sperm for fertilization and disperse by spores that are released into the air (see Fig. 30.1). In these ways, their life cycle is similar to that of the bryophytes. However, the evolution of vascular systems had a major impact on their life cycle because it allowed them to grow tall. Xylem and phloem occur only in the sporophyte generation. This makes sense because gametophytes must remain small and close to the ground to increase the chances of fertilization. Spore dispersal, on the other hand, is enhanced by height, and spore production increases with overall size. An important difference between the life cycles of bryophytes and the spore-dispersing vascular plants is that in bryophytes it is the gametophyte that is the conspicuous photosynthetic generation, while in vascular plants it is the sporophyte generation that dominates in both physical size and photosynthetic output.
To illustrate how the evolution of vascular plants affected plant reproduction, let us examine the life cycle of the bracken fern Pteridium aquilinum, beginning with the largest component, the diploid sporophyte generation (Fig. 30.6). Bracken sporophytes appear to consist entirely of leaves because the stem grows underground. If you examine a bracken leaf closely, you may find tiny brown packets along its lower margin. These are sporangia, and each contains diploid cells that undergo meiosis to generate haploid spores. As in mosses, the spores become covered by a thick wall containing sporopollenin.
The structure of the sporangium aids in spore dispersal. The sporangium wall has a distinct ridge of asymmetrically thickened cells that, upon drying, produce a motion like that of a slingshot that hurls the spores away from the leaf surface to be carried off by air currents. Most spores travel only short distances, but a few may end up far from their parent. Bracken ferns can be found on every continent except Antarctica, as well as on isolated oceanic islands, testimony to the ability of spores to travel long distances.
Spores germinate to produce the haploid gametophyte generation. You have probably never seen a fern gametophyte—or never noticed one. Typical of most ferns, the bracken gametophyte is less than 2 cm long and only one to a few cells thick (Fig. 30.6). The small size of the fern gametophyte has led many to assume that the haploid generation is a weak link in the fern life cycle. In fact, because fern gametophytes tolerate drying out, they often live longer and withstand stressful environmental conditions better than the much larger sporophyte generation.
Most fern gametophytes are capable of producing both male and female gametes, although typically they produce only one type. Chemical signals released by gametophytes producing female gametes (eggs) stimulate gametophytes growing nearby to produce male gametes (sperm) that swim to the egg through a film of water. However, if fertilization does not occur, many fern gametophytes eventually produce both male and female gametes. The union of a male and a female gamete forms a diploid zygote, which is supported by the gametophyte as it begins to grow. Eventually, it forms leaves and roots that allow it to become a physiologically independent, diploid sporophyte.
Ferns, as well as the other spore-dispersing plants (lycophytes, horsetails, and bryophytes), release swimming sperm and thus are able to reproduce only when conditions are wet. What if a plant could eliminate that requirement? Seed plants did just that.
Quick Check 2
In what ways is fern reproduction similar to moss reproduction? In what ways is fern reproduction different from moss reproduction?
30.1.4 In seed plants, the transport of pollen in air allows fertilization to occur in the absence of external sources of water.
What are the advantages of being able to disperse gametes and offspring? Where environments are variable, outcrossing (sexual fusion with a genetically different member of the population) is advantageous because it generates diverse genotypes. Thus, the greater the distance a sperm travels from its parent, the greater the likelihood that it will encounter a genetically distinct egg.
There are also advantages to dispersing offspring. Imagine what would happen if a plant’s spores or seeds simply dropped straight down onto the ground below the parent plant. All the plant’s offspring would be attempting to grow in a very small space that might contain enough nutrients and light for only a few to establish themselves. In contrast, the odds are greater that more of the offspring dispersed by wind or other means will settle in a less crowded spot. In addition, dispersal helps offspring avoid pathogens and parasites. Viruses and bacteria travel easily from individual to individual in a densely packed population. An individual that settles far from the parent plant is less likely to encounter a pathogen.
Let’s return to the question of how seed plants are able to reproduce—to bring male and female gametes together—when conditions are dry. Standing beneath a pine tree on a spring day, you will see the answer to this question all around you: tiny yellow grains that cover the ground and everything else close to the tree. These are pollen grains, and they are produced by all seed plants. It is the evolution of pollen that liberated seed plants from the need to release swimming sperm into the environment. Pollen allows the sperm-producing gametophytes to be transported through the air and thus allows fertilization to take place in the absence of external pools of water. To understand how this happens, let’s look at the life cycle of a pine tree (Fig. 30.7).
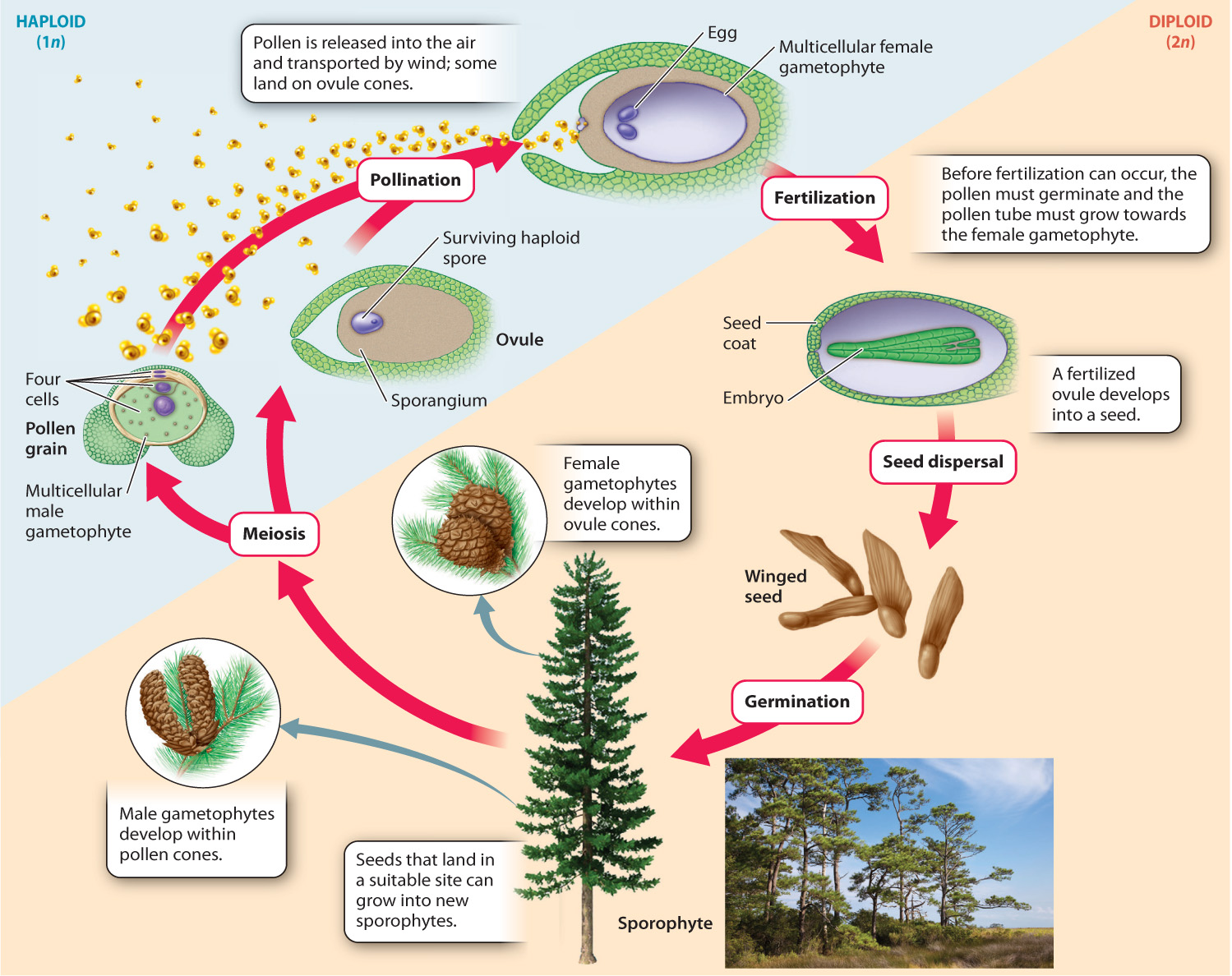
In pines, as in all vascular plants, the conspicuous multicellular generation is the diploid sporophyte. As they mature, pines form reproductive structures called cones. In fact, pines develop two types of cones: ovule cones, which typically occur on upper branches and produce female gametophytes and female gametes, and pollen cones, commonly found in clusters near the tips of branches lower in the tree, which produce male gametophytes and male gametes.
If we examine the pollen cones closely, we see that the helically arranged structures that make up the cone are modified leaves that produce sporangia on their surface. This arrangement, again, is much like that of the bracken fern, whose sporophyte develops sporangia on leaf surfaces. As in ferns, the sporangia in the pollen cones contain cells that undergo meiosis, giving rise to haploid spores whose walls contain sporopollenin.
So far, the pine life cycle closely resembles that of the bracken fern. It is what happens next that sets pines—and all seed plants—apart from other vascular plants. In pines, each spore produced within a pollen cone divides mitotically to form a multicellular gametophyte inside the spore wall. These are male gametophytes, because they produce only male gametes (sperm). Pollen is the multicellular male gametophyte contained within the spore wall.
In pine, the male gametophyte consists of only four cells at the time the pollen is shed from the parent plant. Pine pollen is released into the air and transported by wind. For fertilization to occur, a pollen grain must land on an ovule cone, where the female gametes or eggs are produced.
Ovule cones contain sporangia, each one surrounded by a jacket of protective tissue. In these cones, each sporangium has only one cell that undergoes meiosis and thus produces only four spores. Three of these spores abort spontaneously, leaving a single functional spore inside the sporangium. This spore undergoes repeated mitoses to form a multicellular female gametophyte consisting of a few thousand haploid cells, one or more of which differentiate as eggs. The female gametophyte fills the sporangium, surrounded and protected by the tissues that envelop the sporangium wall. This entire structure is the ovule.
Pollination takes place when the pollen is carried by the wind to an ovule. To reach the egg, however, the pollen must germinate and the male gametophyte must produce a pollen tube that grows outward through an opening in the sporopollenin coat. In pines, pollination occurs as much as 15 months before fertilization. In fact, it is the arrival of the pollen that stimulates the development of the female gametophyte. When the female gametes are ready to be fertilized, the male gametophyte’s pollen tube grows to the female gametophyte, attracted by chemical signals. The sperm travel down the pollen tube and one of them fuses with the egg to form a zygote.
Seed plant reproduction differs from that of spore-dispersing plants in three important ways. The first is that pollination allows fertilization to occur without the male gametes’ ever being exposed to the external environment. The second is that the relationship between sporophyte and gametophyte has been reversed completely from that found in bryophytes. The gametophyte, so prominent in mosses, is reduced to a small number of cells that depend entirely on their parent sporophyte for nutrition (Fig. 30.8). The third difference is that an ovule, when fertilized, develops into a seed. Seeds are multicellular structures that allow offspring to disperse away from the parent plant.
Quick Check 3
How do the male and female gametophyte generations differ in seed plants?
30.1.5 Seeds enhance dispersal and establishment of the next sporophyte generation.
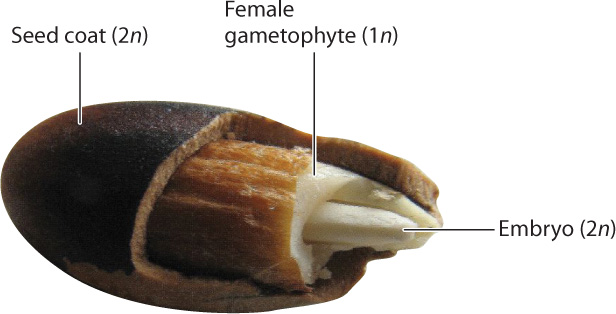
A seed contains structures from three generations (Fig. 30.9). On the outside is the protective seed coat, which is formed from tissues that surround the sporangial wall and thus is a product of the diploid sporophyte. At the center is the embryo, which develops from the zygote and represents the next sporophyte generation. In gymnosperms such as pine trees, the embryo is surrounded by the haploid female gametophyte, which provides the raw materials that support the growth of the embryo.

Compared to a spore, a seed is more likely to form a new, photosynthetically self-supporting plant after dispersal. Because seeds are multicellular structures, they are able to store resources that the embryo can draw on during germination. As they mature, most seeds lose water. And as water content falls, the seeds’ metabolic activity drops to extremely low levels. The combination of low metabolic activity and stored resources allows seeds to survive for long periods, in many cases one to several years, and sometimes much longer.
A final advantage of seeds over spores is that some seeds exhibit dormancy. A dormant seed can delay germination even when conditions for growth, notably temperature and moisture, are favorable. Dormancy prevents seeds from germinating at the wrong time, for example on a warm day at a time of year when the seedlings would be unable to survive. It also prevents seeds from germinating all at once, thus spreading the risk of making the transition from embryo to seedling over a larger time span and range of conditions.
Seeds span over 11 orders of magnitude in size (Fig. 30.10). The 100-nanogram (ng) seeds of orchids are so small (100 ng = 0.0001 mg) that they cannot germinate successfully without obtaining nutrients from a symbiotic fungus. At the other end of the spectrum are the 30-kg seeds of the coco-de-mer, a palm tree found on islands in the Indian Ocean. Large seeds, being better provisioned, are well adapted for the deep shade of a forest understory. However, large seeds typically disperse less far and have shorter life-spans, and they often have little or no dormancy. In contrast, small seeds can persist in the soil until the right combination of conditions triggers germination.
Quick Check 4
Name three advantages of seeds over spores in terms of their ability to disperse.