30.2 FLOWERING PLANTS
Let’s say that you want to send a postcard to a friend, but your only means of doing so is to throw the card into the air and hope the wind carries it to your friend’s front door. How many cards would you have to loft in order to achieve a high probability of success? The answer, of course, is many, and this is precisely the reason that pines generate huge numbers of pollen grains each year. Suppose instead that you can give your card to the letter carrier whose route goes by your friend’s house—your odds of success are now much greater. This analogy gives some idea of a key evolutionary innovation that appeared in the angiosperms. The “letter carriers” in angiosperm fertilization are animals, and the evolution of flowers is what allowed angiosperms to use animals to transport pollen.
Animal pollination increases the efficiency of pollination over what could be achieved by plants such as pines that depend entirely on the wind. That is, a higher proportion of pollen reaches an egg, so the plant can afford to produce less pollen. A second way in which angiosperm reproduction is efficient in its use of resources is that the female gametophyte in angiosperms is even smaller than the female gametophyte in gymnosperms. As a result, fewer resources are wasted when an ovule goes unfertilized. Here, we examine how angiosperms reproduce, beginning with flowers, which when fertilized develop into fruits.
30.2.1 Flowers are reproductive shoots specialized for the production, transfer, and receipt of pollen.

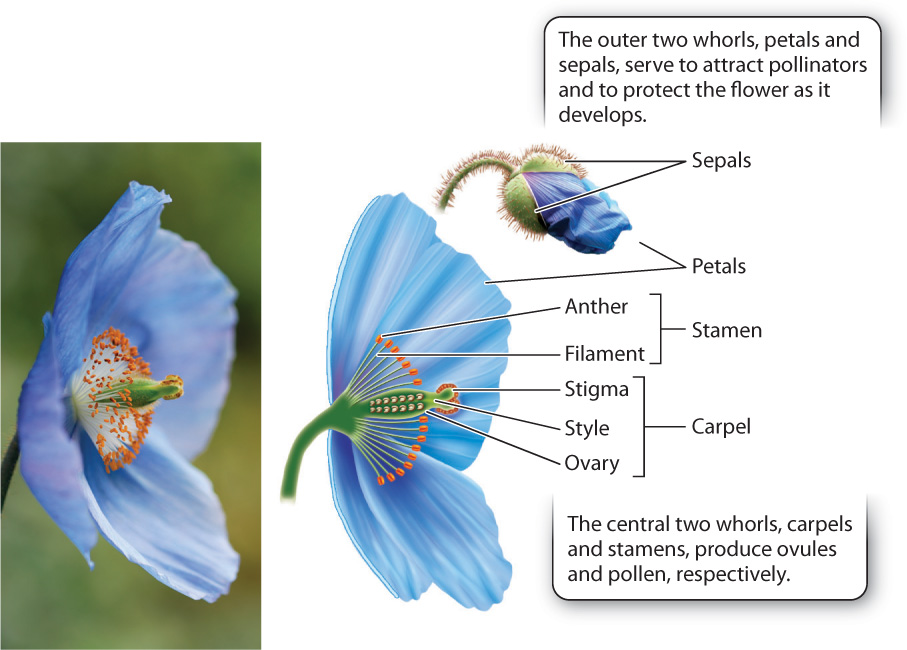
Flowers are spectacularly diverse in size, color, scent, and form (Fig. 30.11). Yet all flowers have the same basic organization: concentric whorls of floral organs (Fig. 30.12; Chapter 20). The center two whorls are made up of ovule-producing carpels and pollen-producing stamens. Here, we see an important difference between angiosperms and gymnosperms. Most flowers produce both pollen and ovules, whereas in gymnosperms, pollen and ovules are produced in separate structures. The significance of this arrangement in angiosperms is that because the sites of pollen and ovule formation are in proximity, a pollinator can deliver pollen to one plant, and take up pollen to carry to another in a single visit. Approximately 12% of flowering plant species produce flowers that form only pollen or ovules, but phylogenetic analysis makes it clear that all these species descend from ancestors whose flowers had both stamens and carpels. Such “unisexual” flowers are often found in plants that have reverted to wind pollination.
Let’s look at the floral organs in more detail, starting with the carpels at the center of the flower (Fig. 30.12). Each flower produces several carpels (typically 3 to 5, but sometimes more than 20), but because the carpels are often fused, it may seem as though there is only one. Each carpel has a hollow ovary at the base in which one to many ovules develop. The ovary protects the ovules from being eaten or damaged by animals. The fact that the ovules are completely enclosed within the carpel is what gives rise to the name “angiosperm,” which is from Greek words meaning “vessel” and “seed.” In gymnosperms, the ovules are not enclosed in a vessel but exposed, and the name, again from the Greek, literally means “naked seeds.”
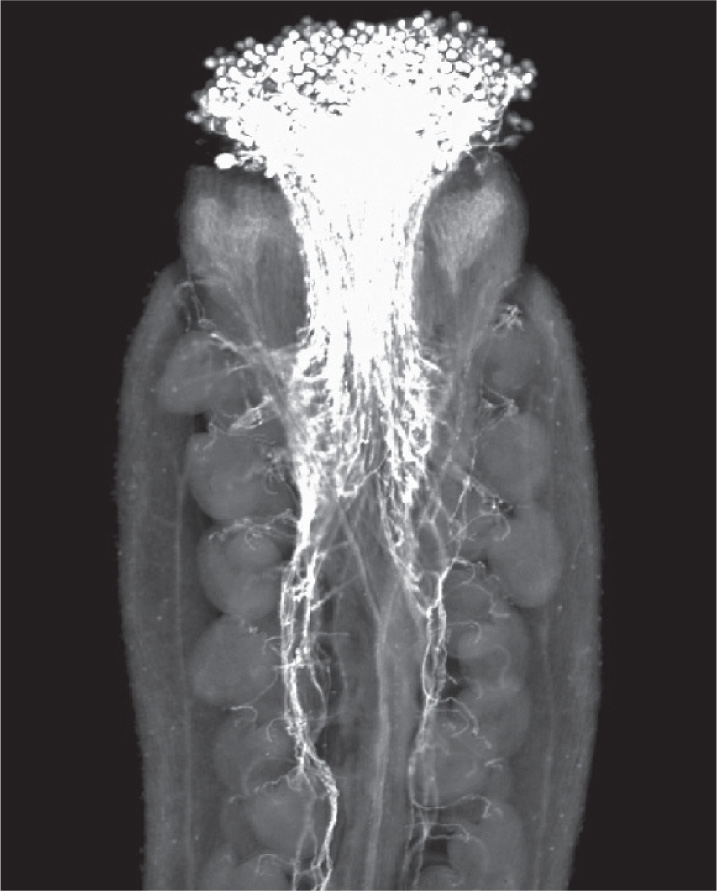
The ovary makes it impossible for pollen to land directly on the ovule surface. Instead, carpels commonly have a cylindrical stalk at the top, called the style. The surface at the top of the style is called the stigma. Pollen is captured on the sticky or feathery stigma, where it germinates and extends its pollen tube down through the style to reach the egg (Fig. 30.13). In some plants the style can be more than 10 cm long—corn silks, for instance, are styles.
Immediately surrounding the carpels are the stamens. A stamen can have a leaflike structure bearing sporangia on its surface, but more commonly it forms a filament that supports a structure known as the anther, which contains several sporangia in which pollen is produced. In most flowers, the anther splits open, exposing the pollen grains. Once exposed, the pollen can come into contact with the body of a visiting pollinator or, in the case of a wind-pollinated species, be carried off by the wind. However, in some plants, for example tomato, small holes open at the top of anthers. To extract the pollen, bees land on the flower and vibrate at just the right frequency to shake loose the pollen inside. Orchids and milkweeds disperse their pollen in a completely different way. Instead of releasing individual pollen grains, they disperse their pollen all together in a package with a sticky tag that attaches to a visiting pollinator.
The outer whorls of the flower produce neither pollen nor ovules but instead contribute to reproductive success in other ways. Most flowers have two outer whorls, of which the outermost is made up of sepals. Sepals, which are often green, encase and protect the flower during its development. In contrast, petals are frequently brightly colored and distinctively shaped. Their role is to attract and orient animal pollinators. In addition to serving as visual cues, petals in many flowers produce volatile oils. These are the source of the distinctive odors, some pleasant, some decidedly not, that many flowers use to advertise their presence to pollinators.
Quick Check 5
What is the name and function of the structures in each whorl of a flower?
30.2.2 The diversity of floral morphology is related to modes of pollination.
The evolutionary histories of the angiosperms and their animal pollinators are closely intertwined. A rapid increase in angiosperm diversity occurred between 65 and 100 million years ago, about the same time as the diversification of bees (Hymenoptera) and butterflies (Lepidoptera). The evolution of flowers allowed animals to specialize in new ways. At the same time, animal pollinators greatly facilitated the movement of pollen between plants within the same population.
So far, we have seen how flowers communicate their presence through both scent and color. For pollination to be reliable, however, it must be in the interest of the animal pollinator to move repeatedly (and with some constancy) between flowers of the same species. Flowers earn fidelity from their pollinators by providing rewards, frequently in the form of food (Fig. 30.14). Many insects consume some of the pollen itself, while many flowers also produce nectar, a sugar-rich solution attractive to bats and birds as well as to insects.


Some species provide rewards other than food. For example, many orchids secrete chemicals that male bees need to make their own sexual pheromones. Other flowers provide an enclosed and sometimes heated chamber in which insects can aggregate. But some flowers do not provide rewards at all: Instead, they “trick” their pollinators into visiting. For example, flowers that look and smell like rotting flesh attract flies, and orchids that look like a female bee even emit similar pheromones to attract male bees (Fig. 30.15). Male bees find these orchids irresistible and, as they attempt to copulate with the flower, deliver and receive pollen.
Producing rewards and attractants such as petals and scents requires resources. Thus, while animals can provide more efficient pollination than can the wind, the resources spent may be wasted if nonpollinating visitors “steal” pollen or nectar. For this reason, many flowers have mechanisms to protect their investments. For example, tubular cardinal flowers provide rewards to hummingbirds able to probe their depths, and snapdragons provide rewards only to insects that are strong enough to push apart their petals.
Many plants have evolved flowers that match rewards and attractants to the metabolic needs and sensory capabilities of their pollinators (see Fig. 30.14). For example, flowers pollinated by larger pollinators such as bats and birds produce copious amounts of nectar. Bat-pollinated flowers tend to be large, white or cream colored, and strongly scented, all traits appropriate to the size and nocturnal habits of their pollinators. The flowers are positioned among branches and leaves in such a way that they can be found by echolocation. In contrast, bird-pollinated flowers are often red, a color insects cannot see, and have no scent. Because interactions between plants and pollinators affect gene flow, they can affect rates of speciation. A recent radiation in columbine occurred as the plants shifted from bumble bees to hummingbirds to hawkmoths as their primary pollinator (Fig. 30.16).
FIG. 30.16Can pollinator shifts enhance rates of species formation?
BACKGROUND Columbines (Aquilegia species) are diverse and have visually striking flowers. Columbine flowers have nectar spurs, which are modified petals that form tubular outgrowths. Blue flowers with short nectar spurs are pollinated by bumblebees, red flowers with medium nectar spurs are pollinated by hummingbirds, and white or yellow flowers with long nectar spurs are pollinated by hawkmoths. The size of the nectar spur corresponds to the length of the tongue of the pollinator.
HYPOTHESIS The recent radiation of columbines corresponds with shifts from short-tongued pollinators (bumblebees) to ones with increasingly longer tongues (hummingbirds, hawkmoths).
METHOD Researchers mapped changes in flower color and nectar spur length onto a phylogenetic tree of columbines. To do this, they assigned each of 25 species examined to one of three groups based on length of tongue, flower color, and most common pollinator (bumblebees, hummingbirds, or hawkmoths). The group is indicated by the color circle at the tip of each branch in the phylogenetic tree in the figure. The researchers then did a statistical analysis that suggested the most likely pollinator type at each node representing an ancestor, also indicated by color circles in the figure. They used this information to infer the history of pollinator shifts during the radiation of this group.
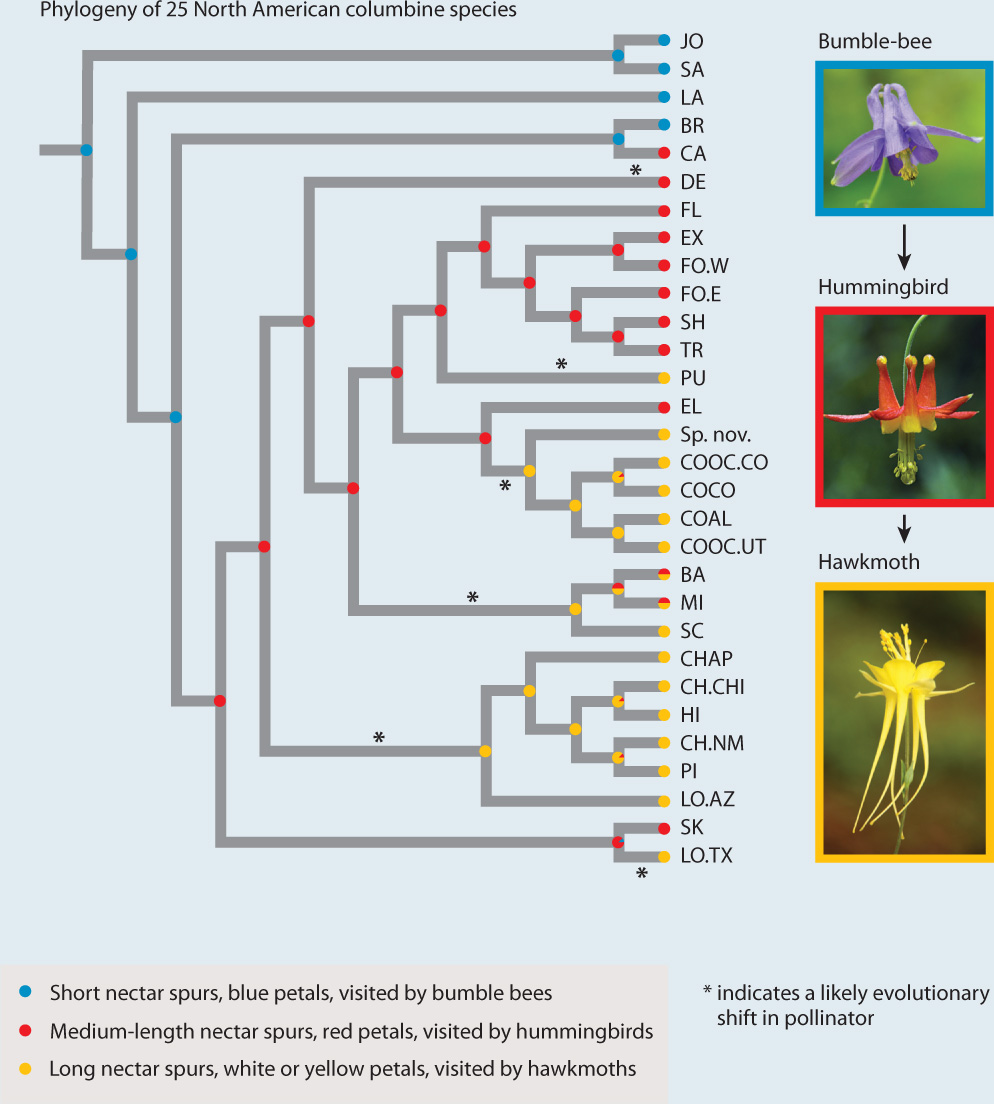
RESULTS Within the recent radiation of columbines, there are multiple instances of shifts from a shorter-tongued to a longer-tongued pollinator, and no reversals.
CONCLUSION The evolution of floral diversity and increasing nectar-spur length in columbines is associated with pollinator shifts. Large changes in nectarspur length occur disproportionately at speciation events, indicating a key role in adaptive radiation.
FOLLOW-UP WORK Developmental studies show that the diversity of nectar spur length evolved as a result of changes in cell shape.
SOURCES Whittall, J. B. and S. A. Hodges. 2007. “Pollinator Shifts Drive Increasingly Long Nectar Spurs in Columbine Flowers.” Nature 447:706–709; Puzey, J. R., S. J. Gerbode, S. A. Hodges, E. M. Kramer, and L. Mahadevan. 2011. “Evolution of Spur Length Diversity in Aquilegia Petals Is Achieved Solely Through Cell Shape Anisotropy.” Proceedings of the Royal Society, London, Series B, 279:1640–1645.
Angiosperms are thought to have evolved in tropical forests. Pollen cannot move freely through the air in the high density of foliage that is present year round in these forests, favoring the evolution of animal pollination. However, as angiosperms diversified and spread around the globe, some evolved wind pollination once again. About 20% of present-day angiosperm species release their pollen into the wind. The reversal to wind pollination is associated with habitats where pollinators are not reliably abundant, such as dry regions.
Wind pollination is most effective in species that are locally abundant. For example, many grasses rely on wind for pollination. Wind pollination is also common in temperate-zone trees, which produce flowers in the early spring, before leaves expand. Wind-pollinated species tend to produce large anthers and stigmas, the latter often branched like a feather duster, well adapted to capture pollen from the air. The sepals and petals of wind-pollinated plants have no attractive role and so tend to be small. Wind-pollinated species produce large amounts of pollen. Hay-fever is an allergic reaction to pollen, virtually all of which comes from wind-pollinated species.
30.2.3 Angiosperms have mechanisms to increase outcrossing.
Plants do not have the elaborate courtship rituals that many animal species use to select an appropriate mate. Nevertheless, angiosperms have a wide range of mating systems. At one extreme are self-compatible species in which pollen and eggs produced by flowers on the same plant can unite and produce viable offspring. Self-compatible species are able to reproduce even when they are physically isolated from other individuals of the same species or when pollinators are rare. Many weedy species and the majority of crops are self-compatible.
At the other extreme are species in which pollen must be transferred between different plants. Approximately half of all of angiosperm species are self-incompatible, meaning that pollination by the same or a closely related individual does not lead to fertilization. Such self-recognition is based on the proteins produced from self-incompatibility genes, or S-genes. If the pollen’s proteins match those of the carpel, the pollen either fails to germinate or germinates but the pollen tube grows slowly and eventually stops elongating. S-genes may have originated as a defense against fungal invaders, but subsequently evolved as a means of promoting outcrossing. Because there can be dozens of S-gene alleles in a population, only pollen transfers between closely related individuals are blocked. Apples are examples of self-incompatible plants, explaining why apple orchards always contain a mixture of different varieties.
30.2.4 Angiosperms delay provisioning their ovules until after fertilization.
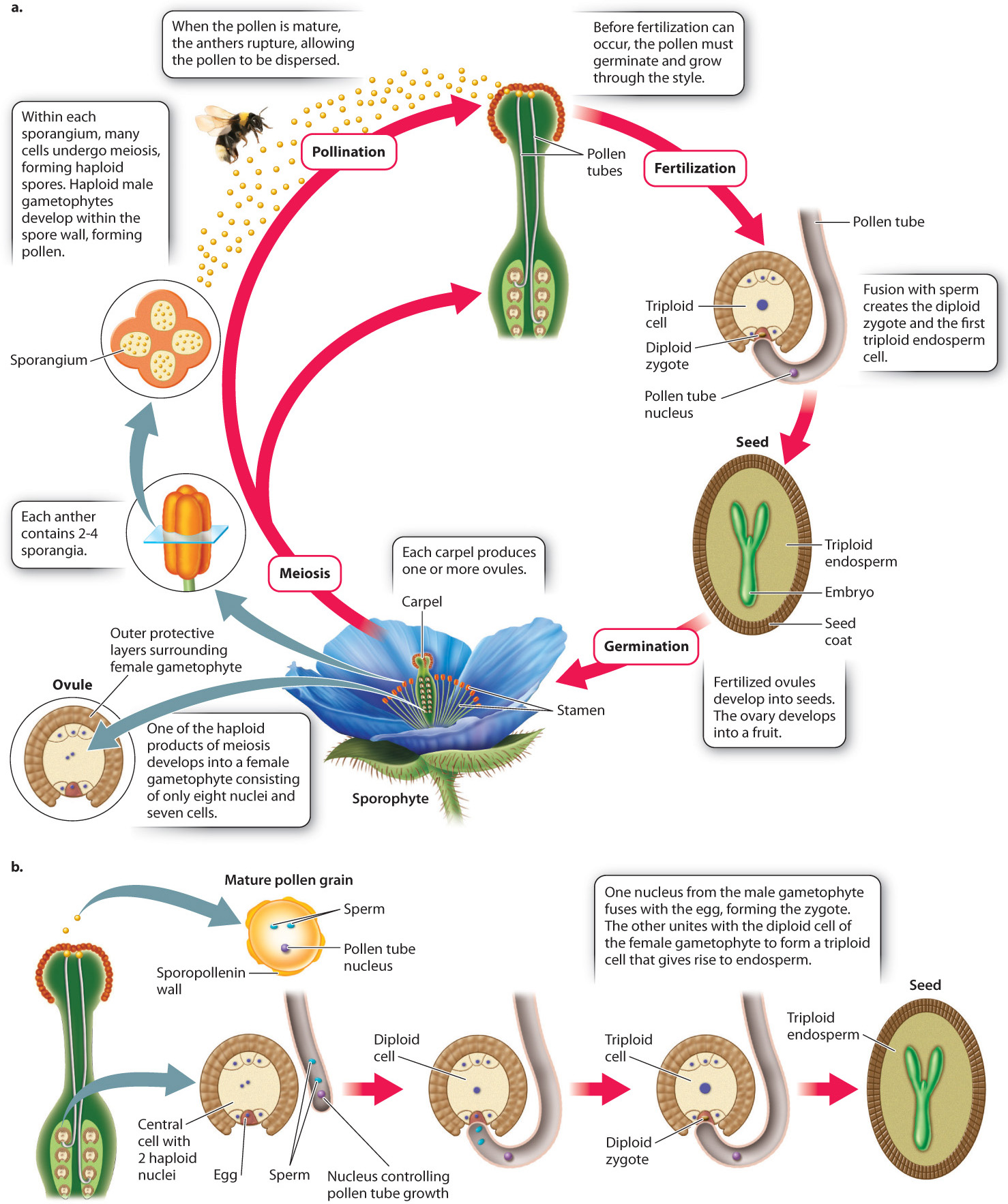
A major trend in the evolution of plants is the decreasing size and independence of the gametophyte generation and the increasing prominence of the sporophyte generation, as we have seen. In angiosperms, this trend is taken even further (Fig. 30.17a). The male gametophyte has only three cells, one of which controls the growth of the pollen tube, while the other two are male gametes or sperm. In most species, the female gametophyte contains only eight nuclei, arranged as six haploid cells plus a central cell containing two nuclei. One of the haploid cells gives rise to the egg, and the two nuclei within the central cell fuse either before or during fertilization to form a diploid cell. The importance of this cell will become apparent. The female gametophyte of angiosperms is too small to support the growth of the embryo, as it does in gymnosperms. How, then, do flowering plants provide nutrition for the embryo to grow?
This is where the diploid cell of the female gametophyte comes into play. During pollination, the two sperm travel down the pollen tube and enter the ovule (Fig. 30.17b). One of the sperm fuses with the egg to form a zygote, just as occurs in all plants. The other sperm, however, unites with the diploid gametophyte cell to form a triploid (3n) cell. This triploid cell undergoes many mitotic divisions, forming a new tissue called endosperm. In angiosperm seeds, it is the endosperm that supplies nutrition to the embryo. The process in which two sperm from a single pollen tube fuse with the egg and the diploid cell is called double fertilization.
What advantage is gained by double fertilization and endosperm formation? In pines and other gymnosperms, the female gametophyte provides food for the embryo. This provisioning occurs whether or not the egg is successfully fertilized, and so is potentially a significant waste of resources. In angiosperms, endosperm development occurs only when fertilization has occurred. In many flowering plants, including corn, wheat, and rice, the mature seed consists largely of endosperm (Fig. 30.18). The embryo itself is small. As the seeds germinate, the embryo draws resources from the endosperm to help it become established. It is no overstatement to suggest that the human population is sustained mostly by endosperm produced in the seeds of cereal crops.
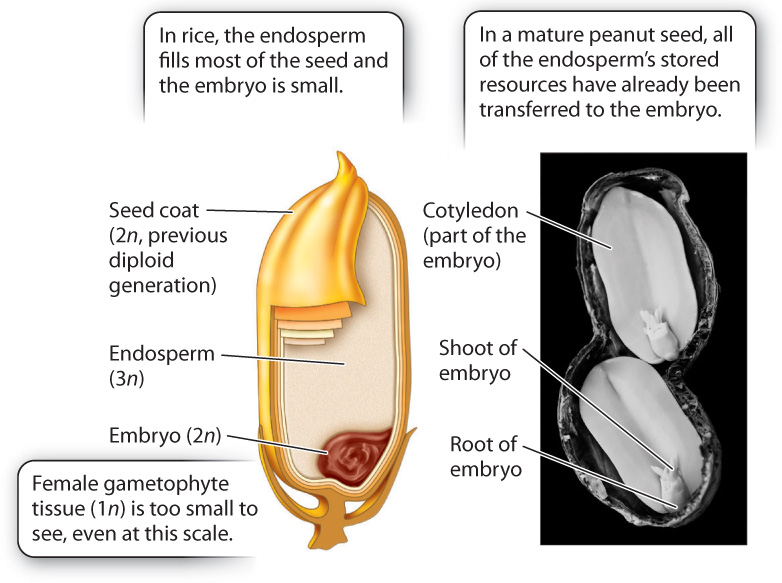
In other angiosperms, growth of the embryo consumes all the endosperm before seed dispersal. For example, a peanut has virtually no endosperm left at the time it is dispersed. All the resources that were previously held within the endosperm have been transferred into two large embryonic leaves, called cotyledons, which form the two halves of the peanut we eat.
30.2.5 Fruits enhance the dispersal of seeds.
The transformation of a flower into a fruit is as remarkable as the metamorphosis of a caterpillar into a butterfly (Fig. 30.19). As the fertilized egg develops to form an embryo and the endosperm proliferates around it, the ovary wall develops into a fruit, stimulated by signaling molecules produced by the endosperm. In a tomato, for example, the fleshy fruit is a mature ovary that encloses the seeds. In some plants, other parts of the flower may also become incorporated into the fruit. For instance, the fleshy part of an apple is formed from the outer part of the ovary combined with the base of the petals and sepals. Other fruits, like pineapple, develop from multiple flowers.

Fruits serve two functions: They protect immature seeds from being preyed on by animals, and they enhance dispersal once the seeds are mature. In the case of fruits dispersed by animals, it is essential that the fruit not be consumed before the seeds are able to withstand a trip through their disperser’s digestive tract. Thus, immature fleshy fruits are physically tough and their tissues highly astringent. As they ripen, the fruits are rapidly transformed in texture, palatability, and color. Ripening converts starches to sugars and loosens the connections between cell walls so that the fruit becomes softer.
In a number of species, including apples, bananas, and tomatoes, a gaseous hormone called ethylene triggers fruit ripening. It is the production of ethylene that explains why placing a ripe apple and an unripe banana together hastens the ripening of the banana. Tomatoes and bananas are usually picked in an immature state and are subsequently ripened by exposure to ethylene. The fruits can therefore be transported while they are still hard and less prone to damage. Because ethylene synthesis requires oxygen, the unripe fruits are typically stored in a low-oxygen environment. Without this precaution, the ethylene produced by the ripening of even a single fruit could set off a chain reaction in which all the stored fruit would ripen at once.

The tremendous morphological diversity of fruits reflects the many ways in which angiosperm seeds are dispersed (Fig. 30.20). The winged fruit of an ash tree is carried away on the wind, and a coconut can float on ocean currents for many months, protected from exposure to salt water by its thick outer husk. The burdock fruit has small hooks that attach to fur (or clothing)—this small hitchhiker was the inspiration for Velcro. Other fruits are not dispersed, but instead open to expose their seeds. For example, as milkweed fruits mature and dry out, they split apart to release hundreds of seeds, each with a tuft of silky hairs that floats them away on even a light breeze. Still others, such as Impatiens (jewelweed), produce fruits that forcibly eject their seeds.
Animals are probably the most important agents of seed transport, in many cases attracted by the nutritious flesh of the fruit. In fleshy fruits, the seeds are protected by a hard seed coat and pass unharmed through the animals’ digestive tract. In some species, this passage actually enhances germination rates. In many cases, however, the seed is consumed, and successful dispersal occurs when the animals gather more seeds than they eat. Squirrels are a good example, storing many acorns that never get eaten. Humans represent an important dispersal agent, responsible for transporting seeds long distances, sometimes far outside their usual range. In some cases, accidentally introduced plant species can disrupt native communities.
Quick Check 6
How does the fruit relate to structures found in the flower?