33.2 BRYOPHYTES
Liverworts, mosses, and hornworts are referred to as bryophytes. They do not form a monophyletic group, but instead a paraphyletic group. Recall from Chapter 23 that a monophyletic group includes a common ancestor and all of its descendants, whereas a paraphyletic group includes a common ancestor and some of its descendants. Nevertheless, liverworts, mosses, and hornworts are discussed as a group and merit their own name because they share so many features despite having evolved independently for hundreds of millions of years.
As the living representatives of the first plant lineages to diverge after plants moved onto land, bryophytes provide us with insights into how plants gained a foothold on the terrestrial environment. This knowledge is particularly useful because the first plants are poorly represented in the fossil record. Only tiny spores and fragments of a cuticle-like covering record these early events.
Fig. 33.1 shows that liverworts, mosses, and hornworts diverged before the evolution of lignified xylem, about 425 million years ago. Thus, bryophytes add to our understanding of the evolutionary history of plants by showing how plants that lack xylem and phloem—key innovations in the evolution of plants—are able to survive in terrestrial environments alongside vascular plants. Of course, we must not lose sight of the fact that bryophytes have continued to evolve as the conditions for life on land have changed over the past 400+ million years. Where bryophytes grow and what they look like have been shaped by their long coexistence with vascular plants.
Quick Check 1
Look at Fig. 33.1 and name the paraphyletic groups indicated by the tree.
33.2.1 Bryophytes are small, simple, and tough.
Mosses are the most widely distributed of the bryophyte lineages and the most diverse, with about 15,000 species. You may have seen moss growing on a shady log or on top of rocks by a stream. However, mosses grow in all terrestrial environments, from deserts to tropical rain forests. Liverworts (about 8000 species) and hornworts (about 100 species) are less widespread and also less diverse. In considering the diversity of these three lineages, let’s start by reviewing some of the features common to all bryophytes.
The most obvious feature that unites the bryophytes is that they are small, most of them only a couple of centimeters in height (Fig. 33.3). The major constraint on bryophyte size is thought to arise from their mode of fertilization. Bryophytes release their sperm into the environment. These sperm must swim through films of surface water or be transported by the splash of a raindrop to meet a female gamete. Sperm can travel in this manner only a relatively limited distance and only if water is present. A few mosses grow to be more than half a meter tall, but these giants of the bryophyte world are found only in the understory of very wet forests.
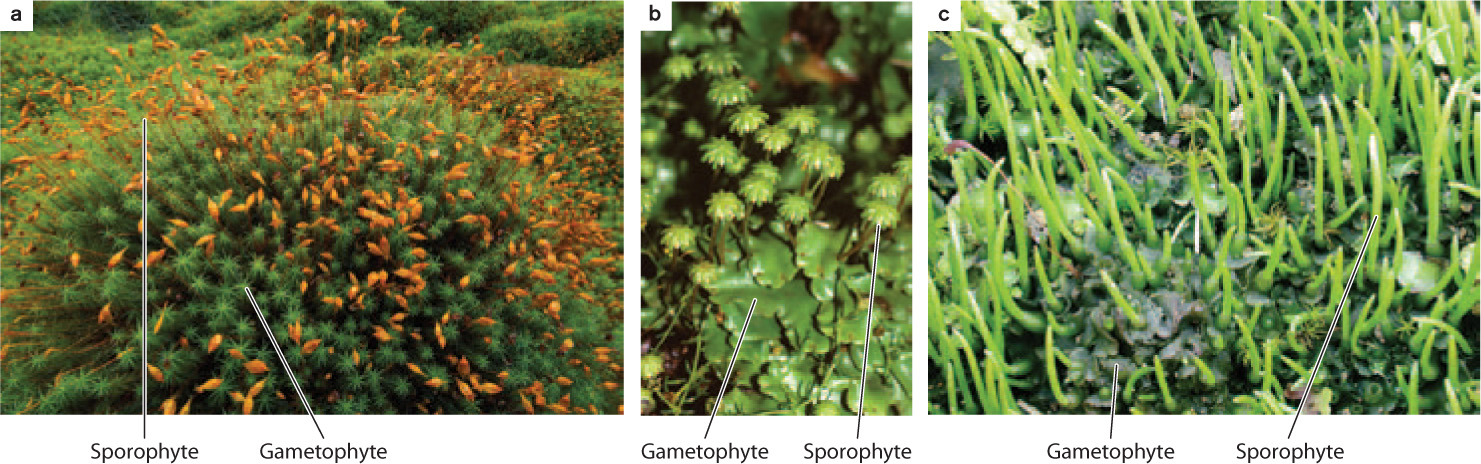
Bryophytes are not only small, they also have simple bodies. Some produce only a flattened photosynthetic structure called a thallus. Others consist of slender stalks and have a leafy appearance. However, these leaf-like structures are quite different from the leaves of vascular plants in that they are only one to several cells thick and lack internal air spaces or a water-conducting system. Both thalloid and leafy species are found in the liverworts, whereas all mosses are of the leafy type and all hornworts are of the thalloid type.
These conspicuous and persistently photosynthetic bryophyte bodies represent the haploid generation. Recall from Chapter 30 that a key innovation that appeared in the land plants is the alternation between a multicellular gamete-producing generation composed entirely of haploid cells and a multicellular spore-producing generation composed of diploid cells. The closest green algal relatives to the land plants have a life cycle in which there is only a single multicellular phase rather than two. The multicellular phase of the green algal relatives corresponds to the haploid, gamete-producing generation of land plants (the gametophyte generation) because both are formed entirely of haploid cells and produce gametes. Thus, the diploid, spore-producing generation (the sporophyte generation) represents a new component of the life cycle that evolved as plants moved onto land.
In bryophytes, the sporophyte remains physically attached to and, in varying degrees, nutritionally dependent upon the gametophyte. In a few liverworts, the sporophyte remains embedded within the thallus, releasing spores only when the gametophyte dies and its body decays. In this case, the multicellular sporophyte generation serves only to amplify the number of haploid spores. In most bryophytes, the sporophyte extends several centimeters above the gametophyte, greatly increasing the chances of spores being dispersed through the air. In a few cases, such as the liverwort Marchantia (Fig. 33.3b), tiny sporophytes grow from the top of upright structures produced by the gametophyte.
In mosses and liverworts, the sporophyte is short lived, drying out after the spores are dispersed. However, in hornworts, the sporophyte can live nearly as long as the gametophyte because it can produce new cells at its base, similar to the way grass blades elongate (Chapter 32). Thus, bryophytes illustrate an important trend in the evolution of plants, an increase in the persistence of the spore-producing generation.
Because bryophytes do not produce lignified xylem conduits, they cannot pull water from the soil. Instead, they absorb water and CO2 through their surfaces, which have little or no waxy cuticle. Bryophytes can absorb enough water to remain metabolically active when the environment is wet, but they must be able to tolerate desiccation when the environment is dry. Thus, while the small size and lack of differentiated structures might suggest that bryophytes are delicate, nothing could be further from the truth. Bryophytes can be found from the equator to both the latitudinal and altitudinal limits of vegetation, and from swamps to deserts. They are the only plants that grow on the Antarctic continent. In fact, the entire flora of that continent consists of approximately 100 moss species and 25 species of liverworts.
Nevertheless, because bryophytes are so small, they are poor competitors for light and space. Instead, they thrive in local environments where roots do not provide an advantage—for example, on the surfaces of rocks. Many bryophytes live on the branches and trunks of trees rather than on the ground. Plants that grow on other plants are called epiphytes (from the Greek words epi, “on,” andphyton, “plant”). Bryophytes are well suited to grow in this way because they are not dependent upon the soil as a source of water.
33.2.2 Bryophytes exhibit several cases of convergent evolution with the vascular plants.
Bryophytes are interesting partly because they are so different from the more familiar vascular plants. However, given that they have long evolved in parallel with vascular plants, it is not surprising that bryophytes have evolved similar solutions to environmental challenges, a process referred to as convergent evolution (Chapter 23). For example, some mosses depend on insects to transport their spores (Fig. 33.4a). These mosses produce brightly colored sporangia, which emit volatile chemicals that mimic compounds released by rotting flesh or herbivore dung. These chemicals attract insects, and the insect legs become covered with spores when they land on the sporangia. When the insect subsequently lands on a real pile of dung, some of the spores fall off into what is, for them, an ideal site for supporting the growth of a new gametophyte.

Another example of convergent evolution is the presence in some mosses and liverworts of cells specialized for the transport of water and carbohydrates. It is clear from the structure of these cells that they evolved independently from the xylem and phloem of vascular plants. In particular, the water-conducting cells in bryophytes do not have lignified cell walls and thus are not sufficiently rigid to pull water from the soil. Nevertheless, water and carbohydrates can move more efficiently from one part of the plant to the other through these elongated cells. Not surprisingly, these internal transport cells are found in the largest of the bryophytes (Fig. 33.4b).
As discussed in Chapter 29, stomata are pores in the epidermis of vascular plants that can open and close, providing an active means of controlling water loss. Stomata also occur on the sporophytes of some mosses and hornworts. It is unknown whether the presence of these stomata is a case of convergent evolution. Answering that question will require further study of the structure and development of bryophyte stomata.
Quick Check 2
If the stomata of bryophytes and vascular plants are not an example of convergent evolution, what is an alternative explanation?
33.2.3 Sphagnum moss plays an important role in the global carbon cycle.
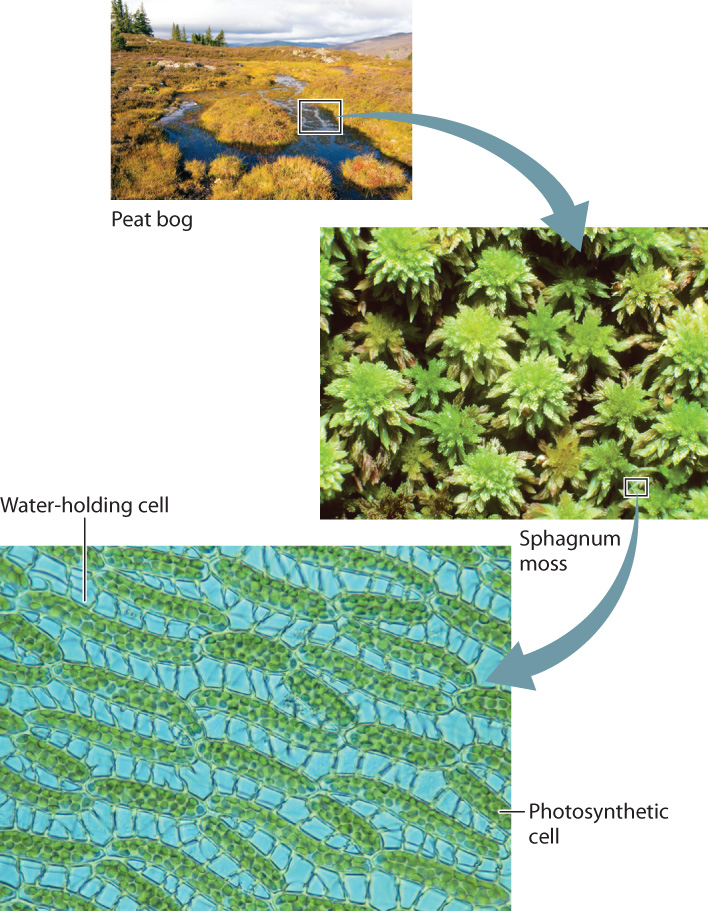
In most ecosystems, bryophytes make only a small contribution to the total biomass. The one exception is peat bogs, wetlands in which dead organic matter accumulates. A major component of peat bogs is sphagnum moss, any of the several hundred species of the genus Sphagnum. These mosses play a key role in creating wet and acidic conditions that slow rates of decomposition. They have specialized cells that hold onto water, much like a sponge, and they secrete protons that acidify the surrounding water (Fig. 33.5). Unlike many plants with roots, sphagnum moss thrives under wet, acidic conditions. In addition, sphagnum mosses produce phenols, organic compounds that slow decomposition under waterlogged conditions.
Peat bogs occupy only 2% to 3% of the total land surface, but they store large amounts of organic carbon—on the order of 65 times the amount released each year from the combustion of fossil fuels. Thus, any increase in the decomposition rate of these naturally formed peats has the potential to increase significantly the CO2 content of Earth’s atmosphere.
What might cause an increase in the decomposition rate? Peat bogs are vulnerable to changes in the water table. As water levels fall, the peat becomes exposed to the air, allowing it to be broken down by microbes. Most peat bogs are located in northern latitudes, where the effects of climate change are predicted to be greatest. If rising summer temperatures result in higher rates of transpiration from surrounding forests, the result could be a lowering of the water table and a relatively rapid release of CO2 from this moss-dominated ecosystem. Thus, peat bogs have the potential to accelerate rates of climate change over the next century.