33.5 ANGIOSPERMS
The evolution of the angiosperms completely transformed life on land. Not only did the total number of plant species increase significantly, but the productivity of terrestrial ecosystems increased as well. As we have seen, the evolution of angiosperms affected the abundance and distribution of other plant groups. In addition, the higher productivity of angiosperm-dominated environments increased the food available for insects, birds, and mammals. All these animal groups diversified along with the flowering plants.
33.5.1 Angiosperm diversity remains a puzzle.
The question of why angiosperms are so diverse has occupied biologists since before Darwin’s time. Despite their efforts, a definitive answer has so far not emerged. Diversity can result from high rates of species formation, and also from low rates of species loss. Interestingly, it may be that low rates of species loss have played an important role in the evolution of the angiosperms. Studies of the fossil record suggest that the rate at which new species appear is comparable for angiosperms and gymnosperms. However, angiosperm species disappear from the fossil record at a lower rate than gymnosperm species.
Flowers may contribute to angiosperm diversity by making it less likely that a rare species will become extinct. Wind-pollinated plants can reproduce only when their populations have a relatively high density. Since there is a high probability that no wind-carried pollen will fall on isolated individuals, species with low population density have a relatively high likelihood of extinction. But animal pollinators actively searching for rare species are much more likely to find them, and so animal-pollinated angiosperm populations can persist at low population densities.
A second feature of angiosperm reproduction that makes it likely that rare species will persist is the extreme reduction in the size of the female gametophyte (Chapter 30). Its small size is possible because the endosperm produced by double fertilization allows angiosperms to delay provisioning their offspring until after fertilization. The price in energy and resources that an angiosperm must pay to take a chance on being fertilized is much lower than in gymnosperms.
Tropical rain forests illustrate how the ability to reproduce at low population density contributes to angiosperm diversity. Rain forests may have several hundred different plant species growing on a single hectare of land, and most of these species are rare.
33.5.2 Early diverging angiosperms have low diversity.
Fig. 33.20 shows a phylogenetic tree of angiosperms. Note that while angiosperms as a whole are remarkably diverse, the early-branching groups of flowering plants are not. Early-branching angiosperms, however, provide important clues to the evolution of flowering plants.
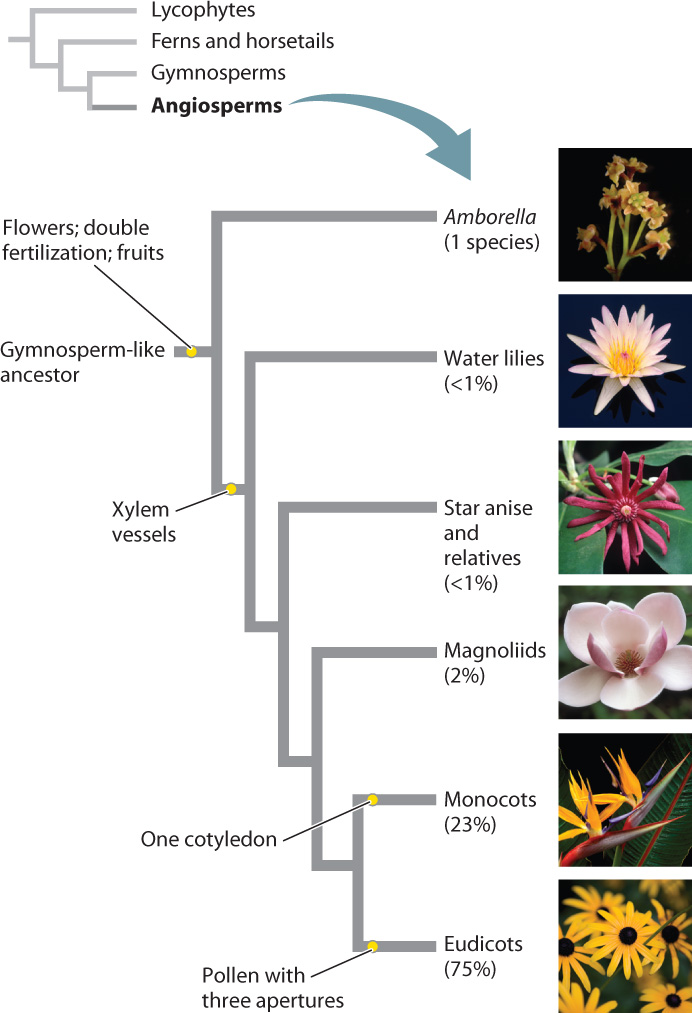
The last common ancestor of angiosperms and living gymnosperms is thought to have lived more than 300 million years ago. In the absence of a closely related sister group, what tools can we use to understand the origin of the angiosperms? The first unambiguous fossils of angiosperms appear in rocks about 140 million years ago. These fossils belong to groups that branch early on the angiosperm phylogenetic tree, but they leave many questions unanswered regarding the ecological context of angiosperm evolution.
To gain further insights into the type of habitat in which the first angiosperms appeared, let’s look at where living plants from the earliest-branching lineages of angiosperms are found today. Most occur in the understory of tropical rain forests; water lilies, which grow in shallow ponds, are one notable exception. This observation suggests that angiosperms may have evolved in wet and shady habitats. How could life in the understory of a tropical forest dominated by conifers have contributed to the evolution of flowers and xylem vessels?
Recall from Chapter 30 that flowers are a plant’s way of enlisting animals to help them reproduce. Brightly colored petals and volatile scents advertise the presence of flowers, while rewards such as nectar encourage pollinators to return repeatedly to individuals of the same species. Because many flowers produce pollen and ovules close to each other, the movement of animals from one flower to another provides an efficient mode of pollen transfer.
Insect pollination may have allowed the first angiosperms to exploit understory habitats of tropical rain forests. Wind provides an effective means of pollen transfer in open habitats or in plants that can produce reproductive structures at the top of the canopy. In the dense understory of a tropical forest, the likelihood that air movements alone would result in fertilization is close to zero. All of the living members of the earliest angiosperm lineages produce flowers that are visited by insects. Fig. 33.21 shows the flower of star anise (Illicium verum), which is visited by beetles and whose fruit is the source of a popular seasoning used in Asian cooking.

Let’s turn now to a second important feature of angiosperms, the formation of wood containing xylem vessels. As described in Chapter 31, most gymnosperms produce only tracheids, a single cell type that serves both the mechanical and the water transport roles of the stem. Angiosperms, in contrast, have two cell types, allowing them to separate the functions of mechanical support and water transport: Vessel elements stack to produce multicellular vessels through which water is transported, and thick-walled, elongate fibers provide mechanical support. This cellular specialization enables angiosperms to produce wood with larger diameter vessels and thus much higher water transport capacity than found in tracheid-only plants. Faster water transport in turn allows greater stomatal opening and thus higher rates of photosynthesis.
In the humid understory of a tropical forest, where transpiration rates are extremely low, there would seem to be little advantage in producing wood with a greater capacity for water transport. In fact, the sole existing species of the earliest-diverging angiosperm lineage—Amborella trichopoda—produces only tracheids. Water lilies also lack vessels, but since they do not produce wood it is less clear whether this is an ancestral or a derived feature. Furthermore, early-diverging angiosperm lineages that do produce vessels do not conduct water at higher rates than those that do not. Thus, xylem vessels in angiosperms appear not to have evolved, at least initially, as a means of enhancing water transport.
Instead, the formation of separate cell types for water transport (vessel elements) and mechanical support (fibers) may have given the early angiosperms greater flexibility in form, allowing them to grow toward small patches of sunlight that filtered through the forest canopy. In the heavily shaded tropical rain forest understory, the more opportunistic growth forms exhibited by early-diverging angiosperm groups, including vines and sprawling shrubs, may have provided a distinct advantage.
The fossil record provides evidence that for the first 30 to 40 million years of their evolution, angiosperms were neither diverse nor ecologically dominant. In fact, fossil pollen indicates that between about 140 and 100 million years ago, gnetophytes diversified just as much as angiosperms. Later in the Cretaceous Period, however, beginning about 100 million years ago, angiosperm diversity began to increase at a much higher rate. Trees belonging to the magnoliids, the branch of the angiosperm tree that today includes magnolias, black pepper, and avocado, emerged as ecologically important members of forest canopies. More important, however, was the divergence of the two groups that would come to dominate both angiosperm diversity and the ecology of many terrestrial environments: the monocots and the eudicots. Together, monocots and eudicots make up more than 95% of angiosperm species. What features may have contributed to their spectacular diversity?
33.5.3 Monocots develop according to a novel body plan.
Monocots or monocotyledons make up nearly one quarter of all angiosperms. Monocots come in all shapes and sizes and are found in virtually every terrestrial habitat on Earth (Fig. 33.22). Coconut palms, hanging Spanish moss (a relative of pineapple), and tiny floating duckweeds, less than 2 mm across, are all monocots, as are the sea grasses of tropical lagoons and the lilies and daffodils in gardens. Some monocots grow in arid regions—including agave, which is used to make tequila. Others, such as orchids, grow primarily as epiphytes in tropical forests. A few monocots form vines—for example, the rattan palms, whose stems are used to produce rattan furniture. Monocots are the most important group of plants in terms of what we eat. Grasses, for example corn, rice, wheat, and sugar cane, are staples in the diets of most people. Other monocots that make their way to our dinner table include banana, yams, ginger, asparagus, pineapple, and vanilla.

Monocots take their name from the fact that they have one embryonic seed leaf, or cotyledon, whereas all other angiosperms have two. However, monocots are distinct in form in so many other ways that one rarely has to count the number of cotyledons to identify a member of this large, monophyletic group. Monocots represent a major evolutionary departure in the way plants build their bodies, in that a vascular cambium is never formed. How has this group been so successful despite the loss of this key innovation? To understand, we first explore in more detail the novel body plan of the monocots.
In monocot leaves, the major veins are typically in parallel and the base of the leaf surrounds the stem, forming a continuous sheath. This type of leaf base means that only one leaf can be attached at any node, consistent with the formation of a single cotyledon. Although monocots do not form a vascular cambium, they can still produce stems that are quite large. For example, both corn and coconut palms are monocots. In monocots, all of the increase in stem diameter occurs in a narrow zone immediately below the apical meristem. As a result of this lateral expansion, the vascular bundles become distributed throughout the stem instead of being arranged in a ring as in all other seed plants.
The lack of a vascular cambium has a profound impact on the way monocots form roots. Because individual roots cannot increase their vascular capacity, monocots continuously initiate new roots from their stems. Thus, the root systems of monocots are more similar to those found in ferns and lycophytes than in other seed plants. Finally, monocot flowers typically produce organs in multiples of 3 (for example, 3, 6 or 9 stamens), whereas eudicot flower organs are most commonly in multiples of 4 or 5.
Monocots have a relatively poor fossil record, in part because they do not produce wood. What factors might have led to such a radical revision in how they build their bodies? The question requires that we look at where and how living monocots grow. One hypothesis is that monocots evolved from ancestors that produced creeping, horizontal stems as they grew along the shores of lakes and other wetlands. Many monocots today, including the earliest diverging groups of monocots, grow in such habitats, and many of the features of the monocot body plan are well suited to environments with loose substrates, flowing water, and fluctuating water levels. For example, their leaf base provides a firm attachment that prevents leaves from being pulled off by flowing water. Furthermore, many monocots produce strap-shaped leaves that elongate from a persistent zone of cell division and expansion located at the base of the leaf blade. By continually elongating from the base, monocot leaves can extend above fluctuating water levels. Finally, it is easier to imagine evolutionary changes that affected the vascular system occurring within an environment that makes only modest demands for water transport.
The environmental context surrounding the evolution of monocots is something we may never know for sure. We thus turn our attention to one of the most diverse groups within the monocots—the grasses. Many grasses produce stems that grow horizontally and branch, allowing them to cover large areas. Grasses have linear leaves that elongate from the base, allowing them to survive grazing as well as fire and drought. Many grasses have evolved the ability to tolerate dry environments by producing roots that extend deep into the soil. In addition, C4 photosynthesis has evolved within the grasses multiple times. As described in Chapter 29, C4 photosynthesis allows plants to avoid photorespiration and thus photosynthesize with greater efficiency. Grasses are among the most successful group of plants, becoming widespread within the past 20 million years as climates changed (Fig. 33.23). Today, nearly 30% of terrestrial environments are grasslands.
FIG. 33.23When did grasslands expand over the land surface?
BACKGROUND Today, prairies, steppes, and other grasslands occur widely in the interiors of continents. Grasses, however, do not fossilize readily. How, then, can we understand how grasslands developed through time?
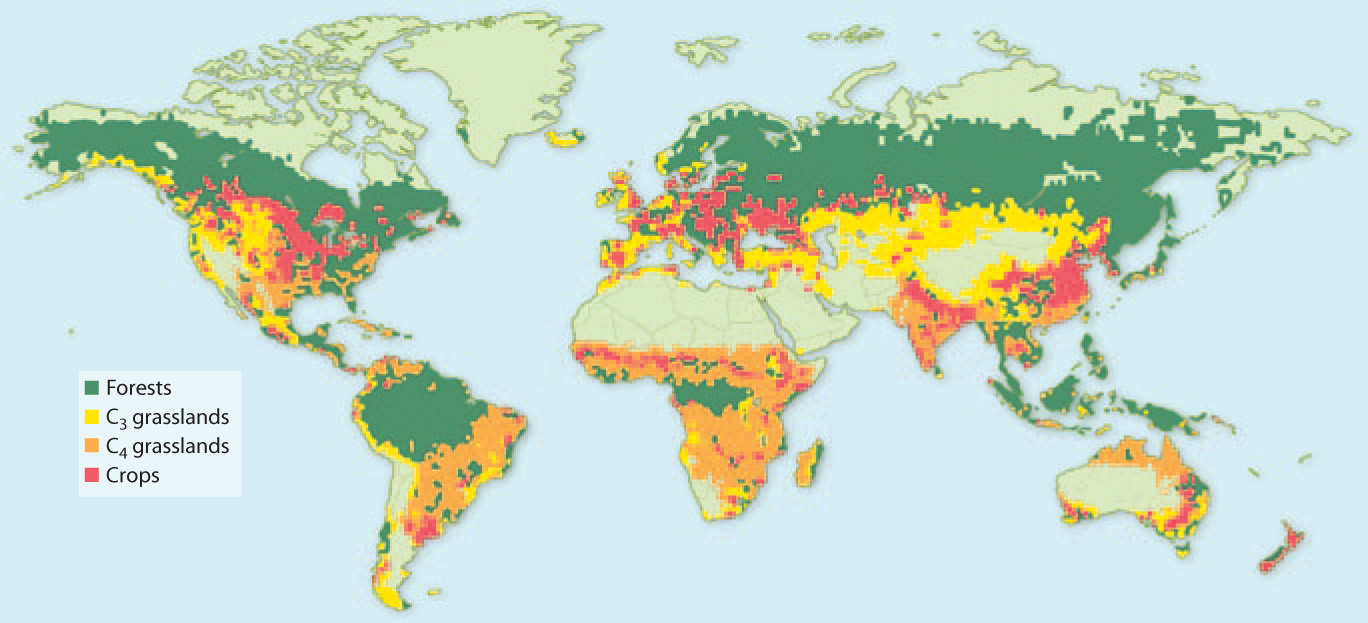
HYPOTHESIS Grasslands expanded as climate changed over the past 50 million years.
OBSERVATIONS AND EXPERIMENTS Grasses commonly make phytoliths, small structures of silica (SiO2) in their cells. These preserve well, providing a direct record of grass expansion. Moreover, mammals that feed on grasses evolved high-crowned teeth, which also preserve well, giving us an indirect record of grassland history. Finally, C4 grasses, which are adapted to hot sunny environments with limited rain fall, have a distinctive carbon isotopic composition imparted by the initial fixation of CO2 by PEP carboxylase (Chapter 29). Measurements of 13C and 12C in mammal teeth, soil carbonate minerals, and more recently, tiny amounts of organic matter incorporated into phytoliths, allow scientists to track the C4 grasslands through time.
RESULTS Studies of phytoliths, mammal tooth structure, and carbon isotopic composition of teeth enamel from the North American midcontinent clearly show that grasslands expanded 15 to 20 million years ago, and C4 grasslands expanded, later, about 6 to 8 million years ago.
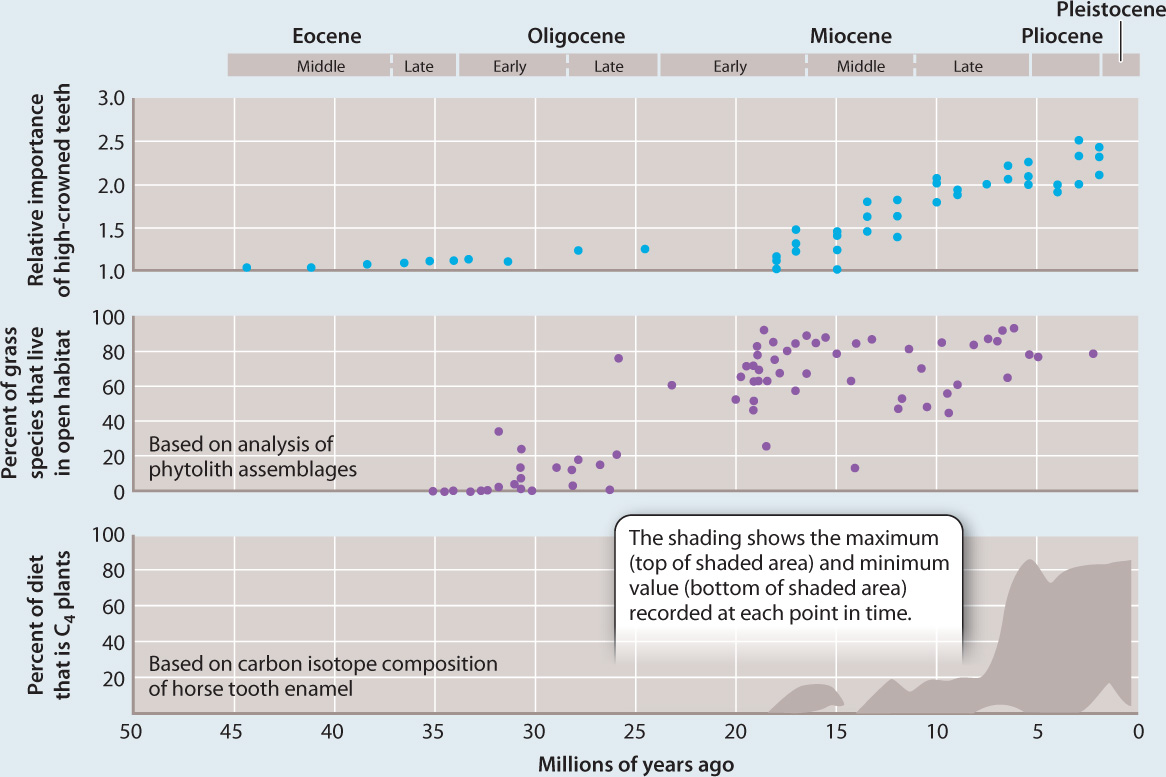
CONCLUSION In North America, grasslands expanded as atmospheric CO2 levels declined and climates became drier. Other continents show evidence of a similar linkage of grassland expansion to climate change.
FOLLOW-UP WORK At present, atmospheric CO2 levels are increasing rapidly, which may affect the competitive abilities of C3 and C4 grasses. Scientists are working to understand how global change will influence grasslands and other vegetation.
SOURCES Stömberg, C. A. E. 2011. “Evolution of Grasses and Grassland Ecosystems.” Annual Review of Earth and Planetary Sciences 39:517-544. Map is from Edwards, E. J., C. O. Osborne, C. A. E. Stromberg, S. A. Smith, and the C4 Grasses Consortium. 2010. “The Origins of C4 Grasslands: Integrating Evolutionary and Ecosystem Science.” Science 328:587-591.
Quick Check 4
What are some of the distinctive features of monocots?
33.5.4 Eudicots are the most diverse group of angiosperms.
Eudicots first appear in the fossil record about 125 million years ago and, by 80 to 90 million years ago, most of the major groups present today can be distinguished. Today, there are estimated to be approximately 160,000 species of eudicots, nearly three-quarters of all angiosperm species (Fig. 33.24). Eudicots are well represented in the fossil record, in part because their pollen is easily distinguished. Each eudicot pollen grain has three openings from which the pollen tube can grow, whereas pollen in all other seed plants has only a single opening. Eudicots take their name from the fact that they produce two cotyledons, whereas monocots produce only one. But because the magnoliids and early angiosperm lineages also have two cotyledons this largest of all angiosperm groups is referred to as the “eu-” or “true” dicots.

Many eudicots produce highly conductive xylem. High rates of water transport, and thus high rates of photosynthesis, may explain why eudicot trees were able to replace magnoliid trees as the most ecologically important members of forest canopies. Today, tropical rain forest trees are an important component of the diversity of eudicots. Important eudicot trees in temperate regions include oaks, willows, and eucalyptus.
At the other end of the size spectrum are the herbaceous eudicots, which represent a substantial fraction of eudicot diversity. Herbaceous plants do not form woody stems. Instead, the aboveground shoot dies back each year rather than withstand a period of drought or cold. At the extreme are annuals, herbaceous plants that complete their life cycle in less than a year, persisting during the unfavorable period as seeds. Annuals are unique to angiosperms and almost entirely occur within eudicots. Why might eudicots be successful as both herbs and trees? One factor is that the ability to produce highly conductive xylem may allow herbaceous eudicots to grow quickly and to produce inexpensive and thus easily replaced stems. Examples of herbaceous eudicots include violets, buttercups, and sunflowers.
The herbaceous growth form appears to have evolved many times within eudicots as tropical groups represented by woody plants expanded into temperate regions. For example, the common pea is an herbaceous plant that resides in many vegetable gardens; its relatives include many tropical rain forest trees. Peas are members of the legume, or bean, family, some of which can form symbiotic interactions with nitrogen-fixing bacteria (Chapter 29). The ability to trade carbon for nitrogen may explain why legumes are important in many habitats. Many of the trees of the African savannas, as well as trees in seasonally dry regions throughout the world, are legumes.
Eudicots are diverse in other ways as well. Most parasitic plants and virtually all carnivorous plants are eudicots, as are water-storing cacti that grow in deserts. Some, such as roses and blueberry, are woody shrubs. Others grow as epiphytes in the canopy of rain forests, and still others, such as grapes and honeysuckle, are vines. Perhaps the most unusual are the strangler fig trees. These begin as epiphytes and then produce roots that descend to the forest floor and fuse to form a solid cylinder that “strangles” their host tree. Finally, eudicots make a significant contribution to the diversity of the dinner table. Apples, carrots, pumpkins, and potatoes are all eudicots, as are coffee, cacao (the source of chocolate), and tea. Many plants with oil-rich seeds such as olives, walnuts, soybean, and canola are eudicots, as are buckwheat and quinoa.
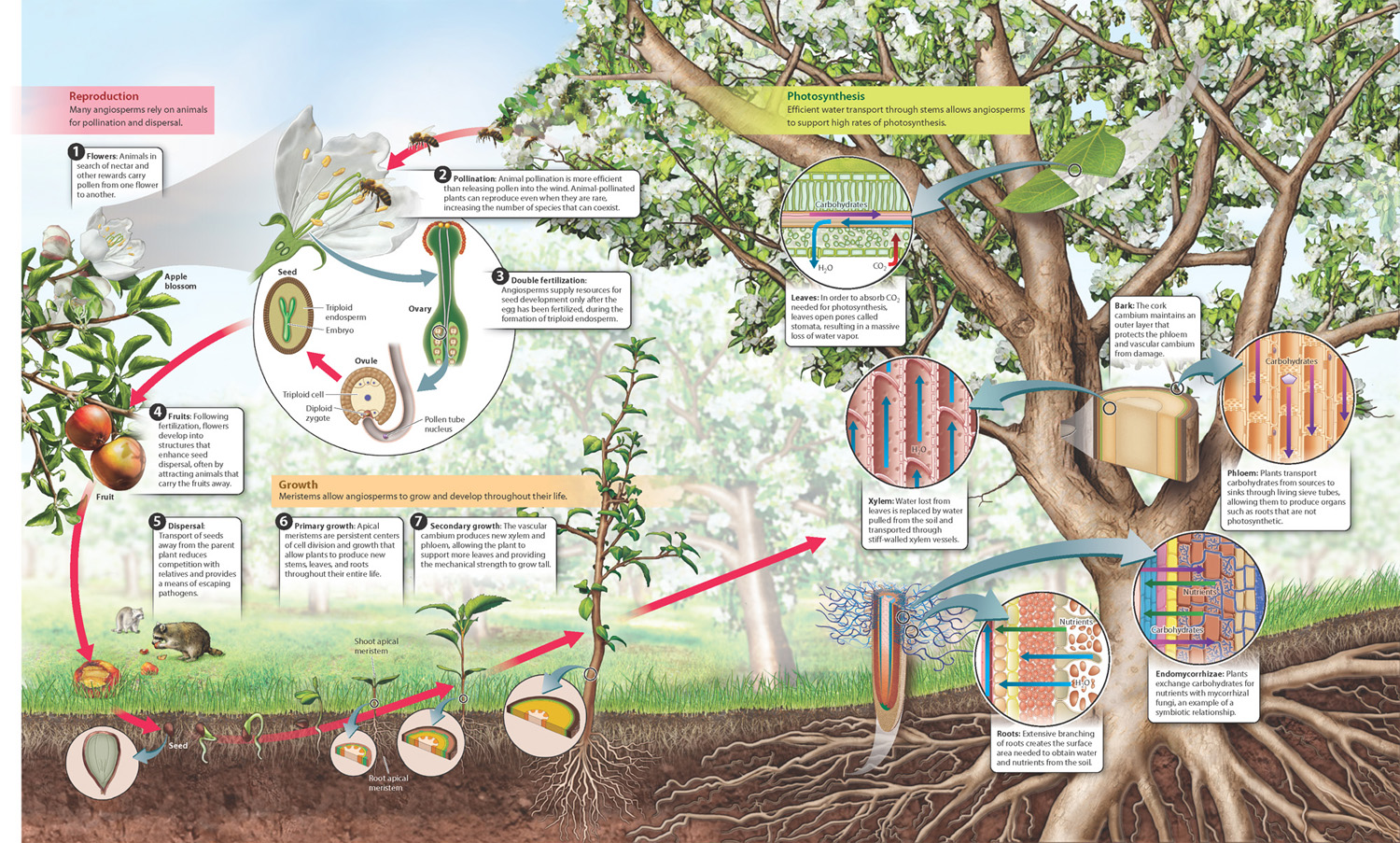
The reproduction, growth, and physiology of angiosperms are summarized in Fig. 33.26.
Case 6 Agriculture: Feeding a growing Population
33.5.5 What can be done to protect the genetic diversity of crop species?
Of the more than 400,000 species of plants on Earth today, we eat surprisingly few. Of those we do eat, nearly all are angiosperms. The only major exceptions are the female gametophyte of a small number of gymnosperms (pine nuts and ginkgos), as well as the young leaves of a few ferns. Most of the plants we eat come from species that are grown in cultivation, many of which have, as a consequence of artificial selection, lost the ability to survive and reproduce on their own. Of the approximately 200 such domesticated species, 12 account for over 80% of human caloric intake. Just three—wheat, rice, and corn—make up more than two-thirds.
Crop breeders select for varieties that grow well under cultivation and are easy to harvest, but a consequence of selective breeding is a decrease in genetic diversity. Thus, not only do we depend upon a small number of species for food, agriculture itself contributes to a narrowing of plant diversity. Because pathogens and pests continue to evolve, any loss in genetic diversity of crop species creates substantial risks. For example, in 1970, the fungal pathogen Bipolaris maydis, a disease-causing organism that had previously destroyed less than 1% of the U.S. corn crop annually, destroyed more than 15% of the corn crop in a single year. The severity of the epidemic was due to the genetic uniformity of the hybrid corn varieties planted at that time. In natural plant populations, genetic variation for pathogen resistance makes it highly unlikely that a newly evolved pathogen strain will be able to infect every plant.
Nicolai Vavilov, a Russian botanist and geneticist, was one of the first to recognize the importance of safeguarding the genetic diversity of both crop species and their wild relatives. In the early twentieth century, he mounted a series of expeditions to collect seeds from around the globe. Vavilov’s observations of cultivated plants and their relatives led him to hypothesize that plants had been domesticated in specific locations (Fig. 33.25), from which they had subsequently expanded as a result of human migration and commerce. Vavilov called these regions “centers of origin,” and he believed that they coincided with the centers of diversity for both the crop species and its wild relatives. In World War II, during the siege of Leningrad (1941-1944) in which more than 700,000 people perished, scientists at the Vavilov Institute sought to protect what was then the world’s largest collection of seeds. Their dedication—at least one of the self-appointed caretakers died of starvation, despite being surrounded by vast quantities of edible seeds—illustrates the priceless nature of the genetic diversity on which our food supply rests.
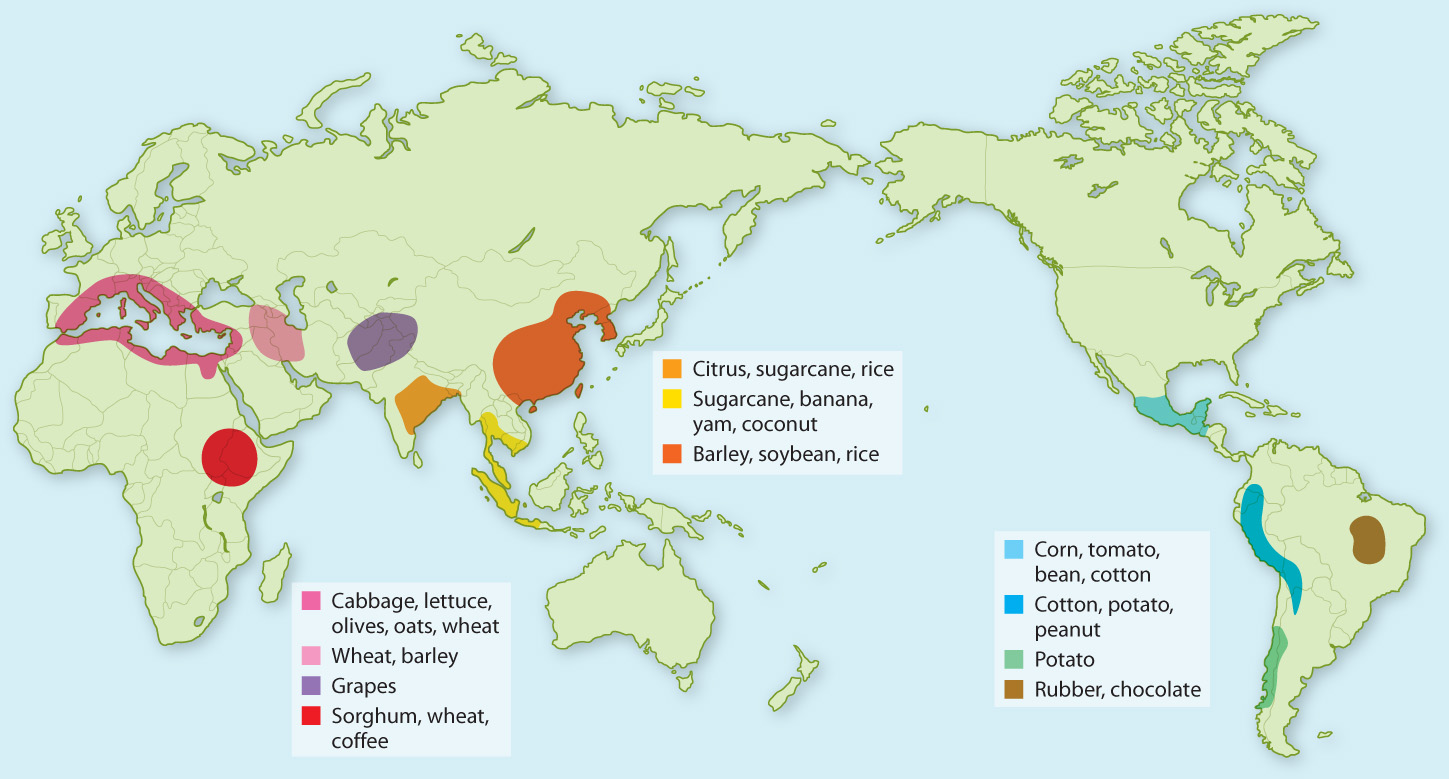
Today, seed banks help preserve the genetic diversity of crop species and their wild relatives. However, seed banks can store only a fraction of the genetic diversity present in nature. To help make up the difference, some have suggested establishing protected areas that coincide with Vavilov’s centers of origin. As we will see in the next chapter, new threats lie just over the horizon. Meeting these threats will require that every genetic resource be brought to bear.
Visual Synthesis: Angiosperms: Structure and Function
Click the image below to view an enlarged version in a new window.