35.3 NEURON FUNCTION
In addition to sharing a common organization, neurons share another feature: They are electrically excitable cells. All neurons encode information by changes in their membrane voltage and transmit information in the form of electrical signals. Nerve signals travel at high speeds, ranging up to 200 m/s (450 mph). Although the speed of nerve signals is fast by biological standards, it is slow by comparison with the speed of electrical signals transmitted by a wire. The speed of signal transmission affects how nervous systems are organized and function.
35.3.1 The resting membrane potential is negative and results in part from the movement of potassium ions.
How are the electrical signals transmitted by animal nervous systems generated within each neuron? The key to producing an electrical signal in a nerve cell is the movement of positively and negatively charged ions across the cell’s membrane. This movement creates a change in electrical charge across the membrane that constitutes the electrical signal.
There is a difference in electrical charge across the cell membrane because unequal numbers of charged ions are located outside and inside the cell. The difference in electrical charge is the voltage and is measured in volts. The charge difference between the inside and outside of a neuron due to differences in charged ions is the cell’s membrane potential. Other cells also have membrane potentials, but only nerve cells and muscle cells (Chapter 37) respond to changes in their membrane potential. Therefore, these cells are considered electrically excitable. Changes in membrane potential provide the basis for transmitting signals between neurons and from neurons to muscle cells.
At rest, when no signal is being received or sent, the cell’s membrane voltage is negative on its inside relative to its outside (Fig. 35.8). The resting membrane potential of the cell is said to be polarized. This means that there is a buildup of negatively charged ions on the inside surface of the cell’s plasma membrane and positively charged ions on its outer surface. The negative voltage across the membrane at rest is referred to as the cell’s resting membrane potential. The resting membrane potential ranges from −40 to −85 millivolts (mV) depending on the type of nerve cell and most commonly is about −65 to −70 mV. The voltage of the cell’s interior can be measured with respect to its outside voltage by small glass electrodes on the inside and outside of the cell.
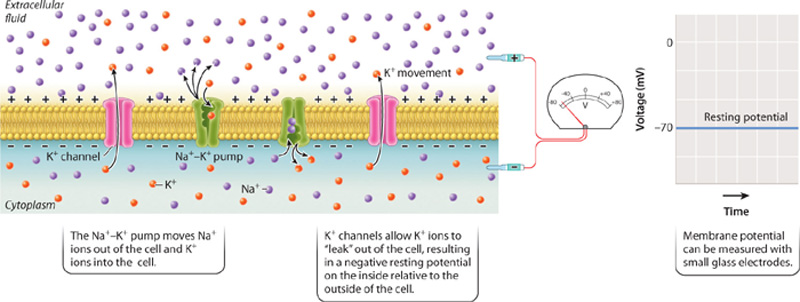
At rest, nerve (and muscle) cells have a greater concentration of sodium (Na+) ions outside the cell and a greater concentration of potassium (K+) ions inside the cell. This distribution of ions results in part from the action of the sodium-potassium pump, discussed in Chapter 5. The sodium-potassium pump uses the energy of ATP to move three Na+ ions outside the cell for every two K+ ions moved in (Fig. 35.8). The action of the pump makes the inside of the cell less positive, and therefore more negative, than the outside of the cell.
The exact value of the resting membrane potential, however, depends on the movement of K+ ions back out of the cell by passive diffusion through potassium ion channels (Fig. 35.8). As discussed in Chapter 5, ion channels are protein pores embedded in the cell membrane’s lipid bilayer. The most important ions that move across the cell membrane are sodium (Na+), potassium (K+), chloride (Cl−), and calcium (Ca2+). When a nerve cell is at rest, more K+ ion channels are open, giving K+ greater permeability compared with all other ions. As a result, K+ ions move out of the cell. The movement of K+ ions from the inside to the outside causes positive ions to build up on the outside of the cell, whereas negatively charged ions (largely Cl– and proteins) remain on the inside of the cell, making the inside of the nerve cell more negative than its outside.
The relative proportion of ions does not by itself determine the resting membrane potential. This is because the number of charged ions that build up at the cell’s surface is a tiny fraction of the total number of charged ions and proteins located inside and outside of the cell. It is the movement of K+ ions relative to other ions, particularly Na+ ions, that largely determines the resting membrane potential.
35.3.2 Neurons are excitable cells that transmit information by action potentials.
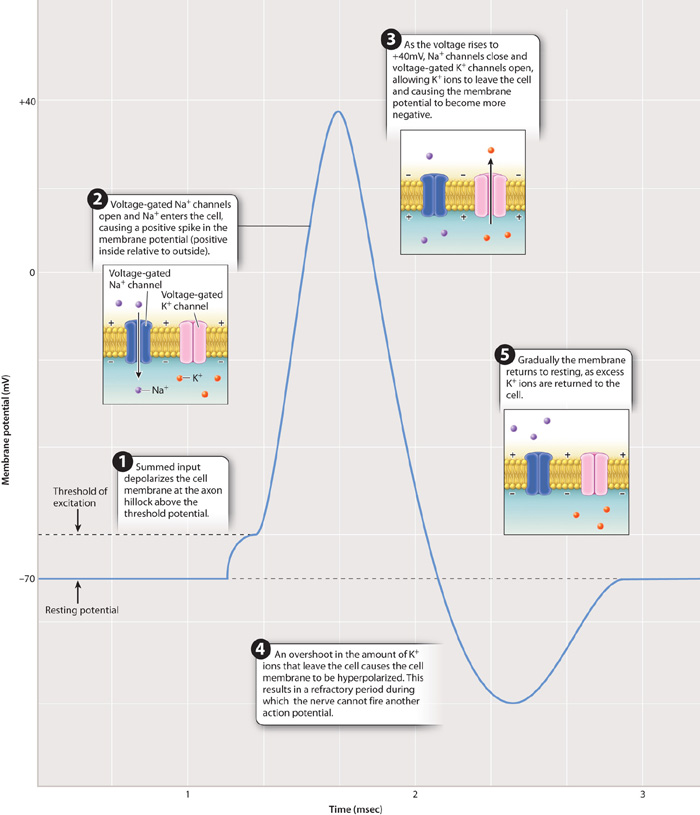
When a nerve cell is excited, its membrane potential becomes less negative—that is, the inside becomes less negative, or more positive, than the outside of the cell. The increase in membrane potential is therefore referred to as a depolarization of the membrane. At the axon hillock of the cell body, this depolarization causes voltage-gated sodium channels to open, allowing Na+ ions to enter the cell. Voltage-gated channels open and close in response to changes in membrane potential. Voltage-gated sodium channels are closed at the resting membrane potential but open rapidly in response to a depolarization of the cell membrane (Fig. 35.9).
The influx of Na+ causes a flow of positive charge, like a current, along the inside of the cell, toward more negatively charged nearby regions. This flow of charge is reduced at longer distances and quickly dissipates so that the membrane potential returns to a resting state unless additional excitatory stimuli further depolarize the membrane.
If the excitatory signal is strong enough to depolarize the membrane of the nerve cell body to a voltage of approximately 15 mV above the resting membrane potential (about –50 mV), the nerve fires an action potential at the axon hillock. An action potential is a rapid, short-lasting rise and fall in membrane potential, as shown in Fig. 35.9. The critical depolarization voltage of –50 mV required for an action potential is the cell’s threshold potential. Nerve cells in different animals have slightly different resting and threshold potentials, but all operate similarly with respect to firing an action potential. When the threshold potential is exceeded, the nerve fires an action potential in an all-or-nothing fashion. This means that once the threshold potential has been exceeded, the magnitude of the action potential is always the same and is independent of the strength of the stimulating input. At this point, a rapid spike in voltage to approximately +40 mV occurs over a very brief time period, 1 to 2 msec.
An action potential results from dramatic changes in the state of voltage-gated Na+ and voltage-gated K+ channels in the axon membrane. As the cell membrane crosses its threshold potential, a large number of voltage-gated Na+ channels suddenly open, allowing Na+ ions to rush inside the cell. The rise in voltage that results causes additional voltage-gated Na+ channels to open. This is an example of positive feedback, in which a signal (depolarization) causes a response (open voltage-gated Na+ channels) that leads to an enhancement of the signal (more depolarization) that leads to an even larger response (more open voltage-gated Na+ channels). During the rising phase of the action potential, voltage-gated K+ channels remain closed, keeping K+ ions inside the cell.
As you can see from Fig. 35.9, the rising phase of the action potential (rapid depolarization) is followed by the falling phase (rapid repolarization). What causes this sudden reversal of the membrane potential? There are two important factors. First, voltage-gated Na+ channels close. These gated Na+ channels do not close in response to the change in voltage, but instead close automatically after a brief period of time. Second, voltage-gated K+ channels open. These channels, like voltage-gated Na+ channels, open in response to the change in voltage, but they are slower to respond than the voltage-gated Na+ channels. As a result of the closing of voltage-gated Na+ channels and the opening of voltage-gated K+ channels, the membrane voltage peaks and then rapidly falls as K+ ions diffuse out of the axon through open voltage-gated K+ channels.
The voltage inside the axon does not return immediately to the resting membrane potential. Instead, it briefly falls below the resting potential (in what is known as hyperpolarization or undershoot), then returns to the resting potential after another few milliseconds as K+ channels close to restore the resting concentration of Na+ and K+ ions on either side of the cell membrane. The continuous action of the sodium-potassium pumps also helps to reestablish the resting membrane potential.
The period during which the inner membrane voltage falls below and then returns to the resting potential is the refractory period (Fig. 35.9). During the refractory period, a neuron cannot fire a second action potential. The refractory period results in part from the fact that when voltage-gated Na+ channels close, they require a certain amount of time before they will open again in response to a new wave of depolarization. In addition, open voltage-gated K+ channels make it difficult for the cell to reach the threshold potential. The duration of the refractory period varies for different kinds of nerve cells but limits a nerve cell’s fastest firing frequency to less than 200 times per second. Most nerve cells fire at lower rates.
It might seem that many Na+ and K+ ions need to diffuse across the axon membrane to produce an action potential. However, only about 1 in 10 million available Na+ and K+ ions need to cross the membrane. Thus, enough ions are always available to generate repeated action potentials.
The nature of the action potential, which is both all-or-nothing and stereotyped, means that the action potential itself contains little information. Most neurons code information by changing the rate and temporal pattern of action potentials that they transmit. Generally, a higher firing frequency codes for a more intense stimulus, such as a brighter light or louder noise, or a stronger signal transmitted by the nerve cell to other cells it contacts.
35.3.3 Neurons propagate action potentials along their axons by sequentially opening and closing adjacent Na⁺ and K⁺ ion channels.
Once initiated, an action potential propagates along the axon. The local membrane depolarization initiated at the axon hillock triggers the opening of neighboring voltage-gated Na+ channels farther along the axon (Fig. 35.10). The inward sodium current depolarizes the membrane above threshold, triggering the opening of nearby voltage-gated Na+ channels still farther along the axon. By this means, the depolarization—the location of the action potential spike—spreads down the axon (Fig. 35.10a). Neighboring voltage-gated K+ channels subsequently also open and close to reestablish a resting membrane potential after an action potential has fired. Action potentials are thus self-propagating. Action potentials propagate only in one direction, normally from the cell body at the axon hillock to the axon terminal. The refractory period following an action potential prevents the membrane from reaching threshold and firing an action potential in the reverse direction (Fig. 35.10b).
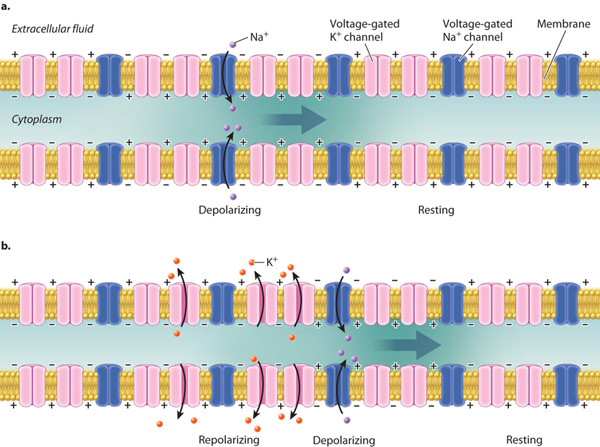
The conduction speed of action potentials is limited by the neuron’s membrane properties and the size of its axon. Yet fast transmission speeds are essential for enabling rapid responses to stimuli that may require communication over long distances within an animal’s body. For example, when you mistakenly touch something very hot, your fingers sense and relay this information rapidly to ensure that you withdraw your hand quickly and avoid worse injury.
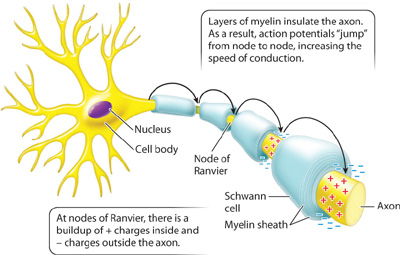
Vertebrate axons, as we have seen, are wrapped in myelin (see Fig. 35.7). The myelin sheath insulates the axon’s membrane, spreading the charge from a local action potential over a much greater distance along the axon’s length than would be possible in the absence of myelin. At regular intervals, the axon membrane is exposed at sites called nodes of Ranvier that lie between adjacent segments wrapped with myelin (Fig. 35.11). Voltage-gated Na+ and K+ channels are concentrated at these nodes. As a result, rather than being conducted in a continuous fashion, as is the case for unmyelinated axons, the action potentials in myelinated axons “jump” from node to node. This saltatory propagation (from the Latin saltus, “jump”) of axon potentials by myelinated axons greatly increases the speed of signal transmission, enabling vertebrate animals to respond rapidly to stimuli in their environment.
Quick Check 2
Multiple sclerosis is a disease in which the immune system attacks and destroys the myelin sheath surrounding nerve cells. What effect would you expect the loss of myelin to have on the speed of nerve impulses?
The British neurophysiologists Alan Hodgkin and Andrew Huxley first worked out the electrical properties of the axon plasma membrane by studying the giant axon in squid that stimulates contractions of their body muscles for swimming (Fig. 35.12). For this work, they shared the 1963 Nobel Prize in Physiology or Medicine along with John Eccles, who worked out the membrane properties of synapses, which we explore next.
FIG. 35.12What is the resting membrane potential and what changes in electrical activity occur during an action potential?
BACKGROUND In order to record the voltage of the inside of a nerve cell relative to the outside, a small electrical recording device, a microelectrode, was inserted into a neuron. The technique is more easily performed on large cells, such as the squid giant axon. This axon, as its name suggests, is quite large, measuring 0.5 mm in diameter (Fig. 35.12a). The large diameter of the axon allows electrical signals to be propagated to the muscles very quickly. Vertebrate neurons are much smaller; they rely on myelin sheaths rather than large size for rapid conduction of electrical signals.
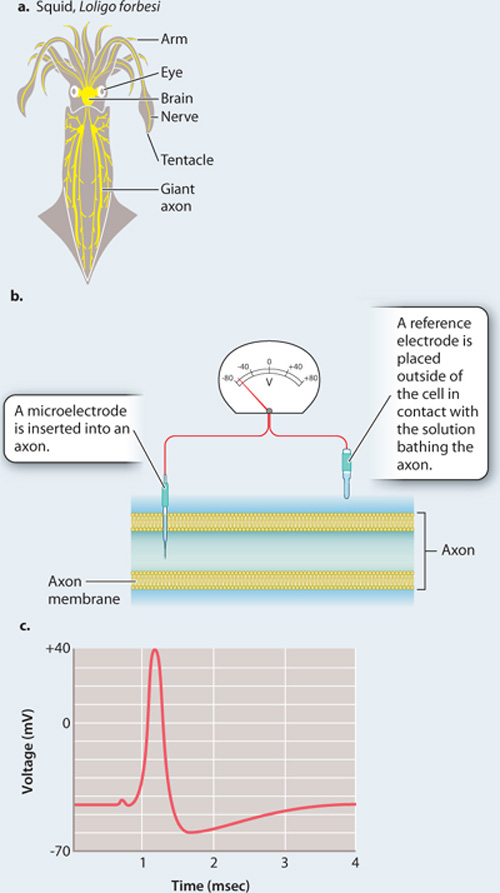
EXPERIMENT In 1939, two British neurophysiologists, Alan Hodgkin and Andrew Huxley, inserted a microelectrode into a squid giant axon with a reference electrode on the outside (Fig. 35.12b). They then used a separate set of electrodes (not shown) to depolarize the cell to threshold, triggering an action potential.
RESULTS Fig. 35.12c is a trace from Hodgkin and Huxley’s 1939 paper showing the resting membrane potential and the course of an action potential recorded by the electrode inside a giant squid axon. Note that the resting potential is negative on the inside of the axon relative to the outside, and that the action potential is a rapid spike in potential, with the inside of the cell quickly becoming positive, then negative again. The large size of the spike was a surprise.
FOLLOW-UP WORK This work was performed before the ion channels responsible for the changes in current across the membrane were identified. Subsequent work focused on identifying these channels and their roles in generating the action potential.
SOURCES Hodgkin, A. L., and A. F. Huxley. 1939. “Action Potentials Recorded from Inside a Nerve Fibre.” Nature 144 : 710–711; R. D. Keynes. 1989. “The Role of Giant Axons in Studies of the Nerve Impulse.” Bioessays 10: 90–94.
35.3.4 Neurons communicate at synapses.
We have seen that nerve cells communicate at specialized junctions called synapses. There are two types of synapse, electrical and chemical. Electrical synapses provide direct electrical communication through gap junctions that form between neighboring cells (Chapter 10). They enable rapid communication but limit the ability to process and integrate information. Electrical synapses are found in the giant axons of squid, as well as in large motor nerves in fish for escape swimming. Electrical synapses can also be found in the mammalian brain. They likely help to speed up information processing, but their role in the brain has not been well studied.
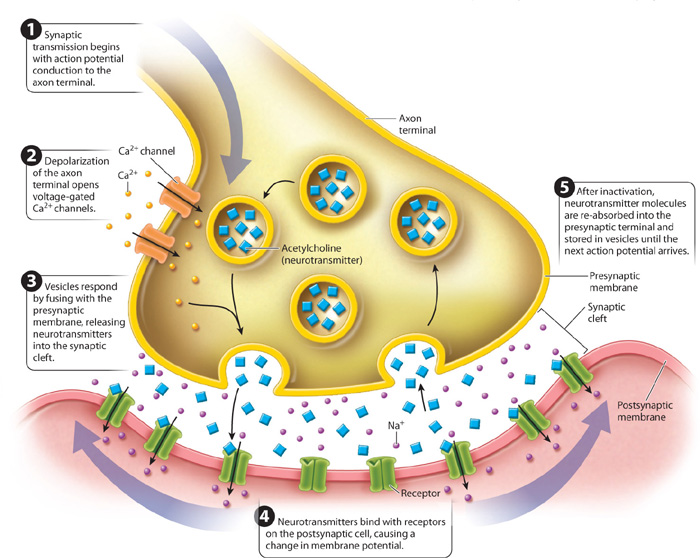
Chemical synapses are by far the more common type of synapse in animal nervous systems. The signals conveyed at chemical synapses are chemicals called neurotransmitters, which are contained within small vesicles in the knoblike axon terminal. When an action potential reaches the end of an axon (Fig. 35.13), the resulting depolarization induces voltage-gated Ca2+ ion channels to open. These channels are found only in the axon terminal membrane. Because of their higher concentration outside the cell, Ca2+ ions diffuse through these channels into the axon terminal. In response to the rise in Ca2+ concentration, the vesicles fuse with the presynaptic membrane and release neurotransmitter molecules into the synaptic cleft by exocytosis. The neurotransmitters diffuse rapidly across the cleft and bind to postsynaptic membrane receptors of the neighboring cell. The binding of neurotransmitters causes a change in the postsynaptic cell membrane potential, allowing the signal to propagate along the next neuron.
The neurotransmitter’s effect on the postsynaptic membrane ends shortly after binding. Neurotransmitters become unbound from the receptor, and neurotransmitters in the synaptic cleft are either broken down by a deactivating enzyme or taken up again by the presynaptic cell and reassembled in newly formed vesicles. The structural and chemical properties of the synapse ensure that communication proceeds in a single direction between nerve cells.
35.3.5 Signals between neurons can be excitatory or inhibitory.
Neurotransmitters bound to postsynaptic membrane receptors can either stimulate or inhibit the firing of action potentials in the postsynaptic neuron. The binding of some neurotransmitters depolarizes the postsynaptic membrane, making it more positive. The positive change in membrane potential is termed an excitatory postsynaptic potential, or EPSP. In contrast, the binding of other neurotransmitters can hyperpolarize the postsynaptic membrane, making it more negative. In this case, the negative change in membrane potential is called an inhibitory postsynaptic potential, or IPSP. Membrane depolarization makes a neuron more likely to respond and transmit an action potential, whereas membrane hyperpolarization inhibits the neuron from sending an action potential.
Excitatory synapses tend to transmit relevant information between nerve cells, and inhibitory synapses often serve to filter out unimportant information. For example, when a grazing gazelle senses a predator, its nervous system must transmit the key sensory information about the presence of a predator but filter out irrelevant information such as the flies buzzing over its back and ears. Inhibitory synapses are also important for controlling the timing of muscle activity required for coordinated movement, discussed in Chapter 37.
How does neurotransmitter binding change the postsynaptic membrane potential? A neurotransmitter binds to receptors that trigger the opening or closing of ligand-gated ion channels in the postsynaptic membrane. The binding of excitatory neurotransmitters causes Na+ channels to open. The postsynaptic membrane becomes depolarized because the opening of Na+ channels allows Na+ ions to enter the cell. In contrast, the binding of inhibitory neurotransmitters causes Cl–, or sometimes K+, channels to open. Cl– ions diffuse into the cell or K+ ions diffuse out of the cell through these channels, causing the membrane potential to become more negative, or hyperpolarized, than the outside.
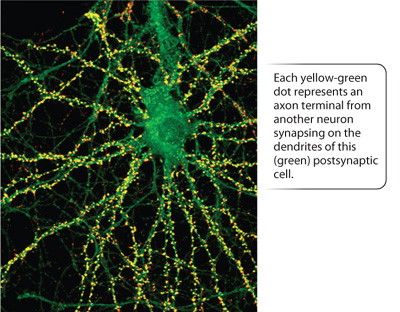
Different types of nerve cell contain different neurotransmitters, but each type of nerve cell releases only one type of neurotransmitter. A particular type of nerve cell exerts either excitatory or inhibitory effects on neighboring cells, but not both. In contrast, postsynaptic membranes typically have multiple types of membrane receptors that bind different kinds of neurotransmitters. Thus, postsynaptic cells can receive both excitatory and inhibitory signals. Commonly, the dendrites or cell body of a single postsynaptic nerve cell make connections to hundreds or even thousands of axons from other nerve cells (Fig. 35.14).
The postsynaptic nerve cell sums the excitatory and inhibitory synaptic inputs (the summed EPSPs and IPSPs) that it receives through its dendrites and cell body (Fig. 35.15). If the sum of synaptic stimulation results in a membrane potential that exceeds threshold at the axon hillock, the neuron fires an action potential, communicating an impulse to cells that it in turn contacts. If the sum of inputs does not exceed threshold, no action potential fires (Fig. 35.15a).
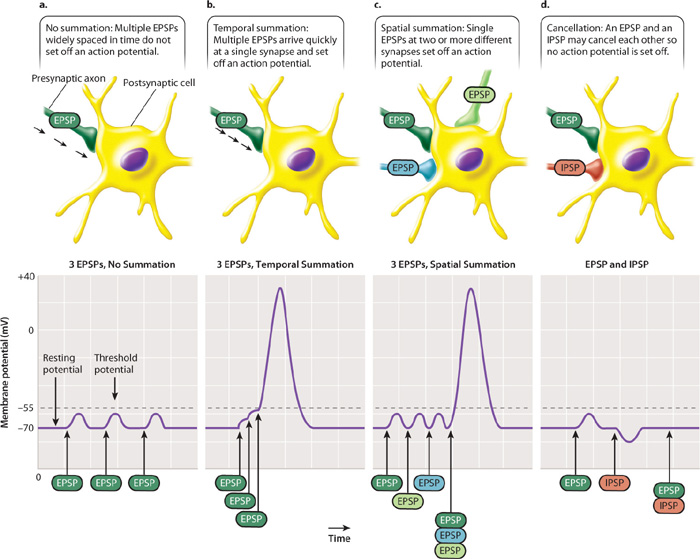
EPSPs and IPSPs can be summed over time and space. When summed over time, the frequency of synaptic stimuli determines whether the postsynaptic cell fires an action potential (Fig. 35.15b). This is referred to as temporal summation. When summed over space, the number of synaptic stimuli received from different regions of the postsynaptic cell’s dendrites determines if the cell fires an action potential (Fig. 35.15c). This is referred to as spatial summation. Sometimes, excitatory and inhibitory signals may cancel each other out (Fig. 35.15d). Temporal and spatial summation of EPSPs and IPSPs are the fundamental forms of information processing carried out by the nervous system. They provide mechanisms for determining whether a particular sensory stimulus is responded to or ignored. The role of temporal and spatial summation is explored further in Chapter 36.
More than 25 neurotransmitters are now recognized, and more are likely to be discovered. The amino acids glutamate (excitatory), glycine (inhibitory), and GABA (inhibitory) are key neurotransmitters that operate at synapses in the brain. The related amino acid derivatives dopamine, norepinephrine, and serotonin are also neurotransmitters acting in the brain. Simple peptides serve as neurotransmitters for sensory neurons. More recently, two gases, nitrous oxide and carbon monoxide, have been discovered to function as messengers between nerve cells in various regions of the nervous system, even though they do not behave as standard neurotransmitters that bind to membrane receptors.
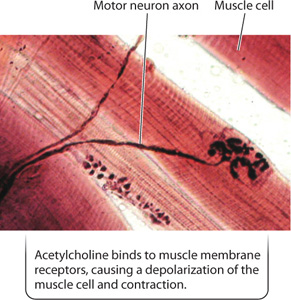
Acetylcholine is one of the key neurotransmitters produced in a variety of nerve cells. It is the excitatory neurotransmitter released by motor neurons to stimulate muscle fibers at a type of synapse called the motor endplate (Fig. 35.16). All vertebrate motor synapses rely on acetylcholine and are therefore excitatory only. The release of acetylcholine produces a depolarization of the muscle cell membrane, causing the muscle to contract.
Quick Check 3
Acetylcholinesterase is the enzyme that breaks down and inactivates acetylcholine. Some nerve gases used as chemical weapons block acetylcholinesterase. What effect would such nerve gases have on muscle contraction?