36.3 SENSING GRAVITY, MOVEMENT, AND SOUND
The ability to detect motion, orient with respect to gravity, and hear all depend on specialized mechanoreceptors called hair cells that sense movement and vibration. They are found in fishes and amphibians, in which they detect movement of the surrounding water, and in many invertebrates, in which they sense gravity and other forces acting on the animal. They are also found in the ears of terrestrial vertebrates, in which they sense sound, body orientation, and motion.
In all these cases, hair cells sense mechanical vibrations. Movement of small nonmotile cell-surface projections called stereocilia causes a depolarization of the cell’s membrane by opening or closing ion channels. Despite their name, stereocilia are more similar to microvilli than to cilia, which unlike stereocilia can move on their own (Chapter 10). Stereocilia contain actin filaments but lack the microtubules and dynein motor proteins of motile cilia. Hair cells themselves do not fire action potentials but instead release neurotransmitters that bind to receptors in adjacent sensory neurons, altering their firing rate.
36.3.1 Hair cells sense gravity and motion.
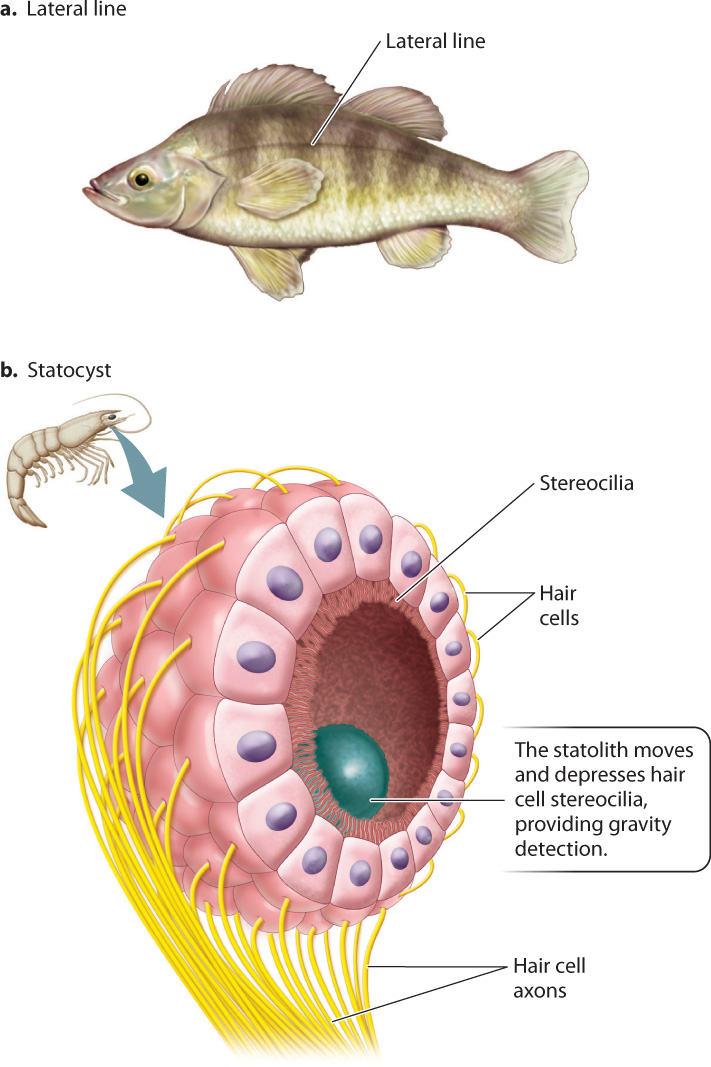
Hair cells detect gravity and motion in many animals. Hair cells contained in the lateral line system of fish and sharks sense water vibrations that indicate nearby threats or prey, as well as their own motion (Fig. 36.6a). The lateral line is a sensory organ along both sides of the body that uses hair cells to detect movement of the surrounding water.
A sense of gravity helps animals to orient their body within the environment, providing a sense of “up” and “down.” Even buoyant aquatic animals depend on a sense of gravity to keep oriented to the water’s surface or within the water column. Gravity-sensing organs called statocysts are found in most invertebrates (Fig. 36.6b). The statocyst is made up of an internal chamber lined by hair cells with stereocilia that project into the chamber. Small granules of sand or other material form a dense particle called a statolith that is free to move within the statocyst organ. By pressing down on hair cells at the “bottom” of the chamber, the statolith activates those cells, indicating the direction of gravity. Statoliths help anemones and jellyfish orient themselves in the water and direct their tentacles. When statoliths of lobster and crayfish are replaced by magnetic particles and subjected to an experimental magnetic field, the lobsters and crayfish swim upside down or on their sides in response to the experimental change in the direction of the magnetic field.
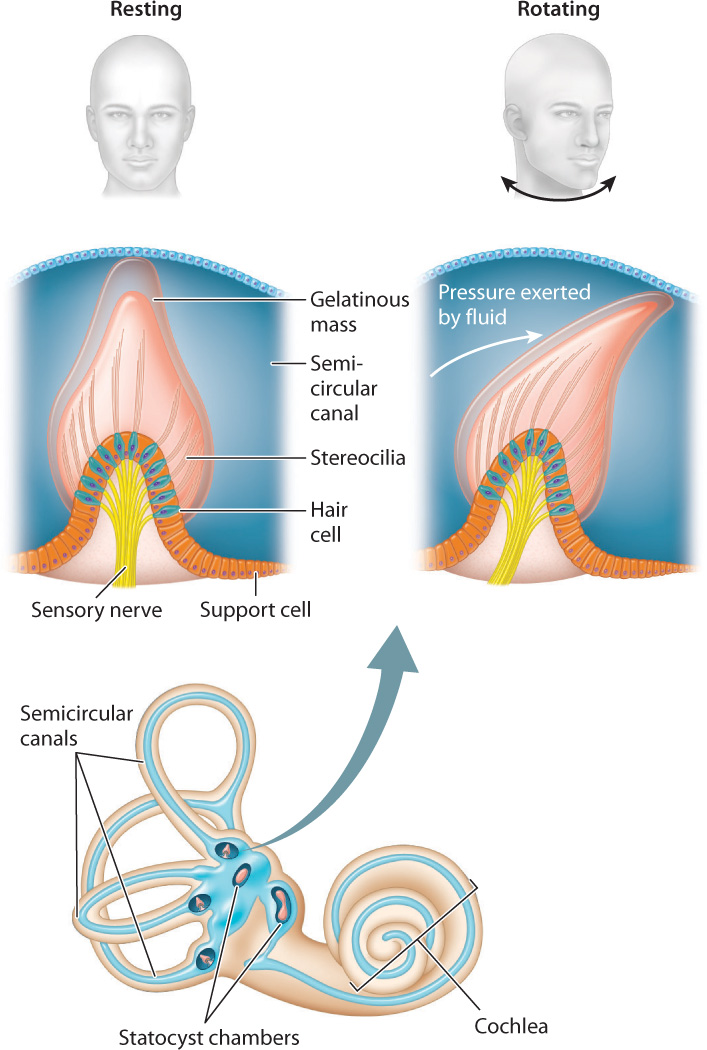
The mammalian inner ear contains organs that sense motions of the head and its orientation with respect to gravity. These organs make up the vestibular system (Fig. 36.7), which consists of two statocyst chambers and three semicircular canals. These statocyst chambers are similar to those of invertebrates, providing a sense of gravity and of body orientation with respect to motion. Hair cells located within the semicircular canals sense angular motions of the head in three perpendicular planes, providing a sense of balance. When the head rotates, the motion of gelatinous fluid in the semicircular canals is accelerated, deflecting the stereocilia of the hair cells to activate sensory neurons. The brain interprets differences in the motion of the fluid with respect to the hair cells among the three semicircular canals to resolve angular motions of the head in any direction. As a result, mammals and birds react much faster to head motion than to visual cues, and that ability helps them to stabilize their gaze.
Quick Check 3
After you spin in place or take a ride on a merry-go-round, why are you unstable when you try to walk?
36.3.2 Hair cells detect the physical vibrations of sound.
What our ears interpret as sound are alternating waves of pressure traveling through the air. The frequency of the waves—the number of waves per second—determines the pitch of a sound, and the amplitude, or height, of the waves determines its loudness. Sound waves cause the stereocilia of hair cells to bend, and the hair cells become excited, depolarizing and releasing neurotransmitters. The process sounds simple, but how can the bending of stereocilia produce all the variations in pitch and loudness you hear in the world around you?
Insects hear by sensing airborne vibrations with small hairs on their antennae or other regions of their body. Hairs of different length detect different frequencies because the shorter hairs are stiffer than long hairs and vibrate at higher frequencies. Frequency is measured in Hertz (Hz), or cycles per second. Male mosquitoes have small antennal hairs that vibrate in response to the 500-Hz hum of a female mosquito’s flight, which attracts them to the female to mate. Many insects also have specialized ears that sense sound by means of a tympanic membrane. The tympanic membrane is a thin sheet of tissue at the surface of the ear that vibrates in response to sound waves, amplifying airborne vibrations. The vibration excites hair cells attached to the inside of the tympanic membrane, and these hair cells in turn excite other neurons to fire action potentials. Crickets and other singing insects sense the chirping or buzzing of potential mates by their ears.
Hearing is most widespread and developed in terrestrial vertebrates. The ears of amphibians, reptiles, and birds have a simple external tympanic membrane that vibrates when sound waves strike its surface. This membrane is similar to the tympanic membrane of insects but evolved independently and therefore is a case of convergent evolution. Terrestrial vertebrate ears can detect a broad range of sound frequencies. For humans, the range of audible frequencies is from 20 to 20,000 Hz. For dogs, the audible range is 70 to 44,000 Hz, and some rodents can hear up to 80,000 Hz. Birds generally hear in the same frequency range as mammals—one reason bird songs are pleasing to our ear. Frogs and reptiles generally hear at lower frequencies than mammals and birds. Vertebrate ears can also make fine distinctions between close frequencies and can detect softer sounds because external structures amplify the sounds before they reach the hair cells.
The ears of mammals have an external structure, the pinna, that enhances the reception of sound waves contacting the ear. Notice the heightened sensitivity that this structure provides for hearing when you cup your hands behind your ears. It is no coincidence that dogs and rabbits perk up their ears when alerted by a sound: This action orients them to the direction of the sound’s source.
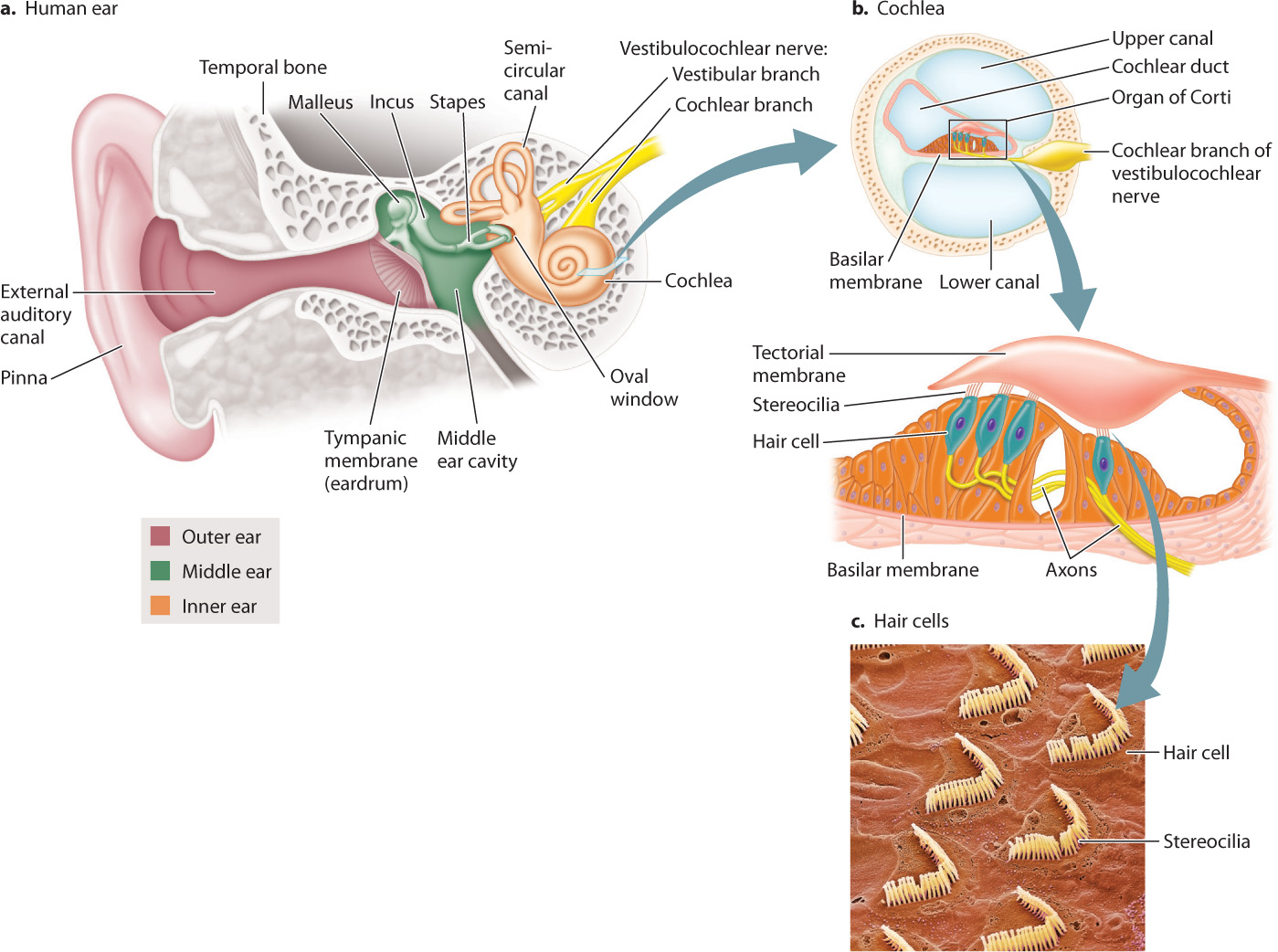
Fig. 36.8 shows the structure of the human ear. The pinna is part of the outer ear, which also includes the ear canal and tympanic membrane, or mammalian eardrum, which transmits airborne sounds into the ear. The middle ear contains three small bones or ossicles, called the malleus, incus, and stapes, which amplify the waves that strike the tympanic membrane. The stapes connects to a thin membrane called the oval window of the cochlea in the inner ear. The cochlea (which means “snail” in Latin) is a coiled chamber within the skull that contains hair cells that convert pressure waves into an electrical impulse that is sent to the brain.
The mammalian eardrum evolved from the tympanic membrane of reptiles and earlier amphibians. The three middle-ear bones are also evolutionarily related to three bones in reptiles, but only the stapes functions in reptiles to transmit vibrations of the tympanic membrane to the inner ear. The malleus and incus help support jaw movements during feeding in reptiles. During the evolution of mammals from their reptilian ancestors, these two bones were incorporated together with the stapes to form the middle-ear bones that transmit sounds from the eardrum to the inner ear.
The process of hearing can be separated into three stages:
- Amplification. Sound vibrations received by the outer ear are transmitted by the eardrum and amplified by the three bones in the middle ear (Fig. 36.8a). Through piston-like actions, the stapes transmits the energy of its movements to vibrations of the oval window of the cochlea in the inner ear. Vibrations transmitted from the eardrum through the middle ear to the oval window are amplified more than 30 times because of the larger size of the eardrum compared to the oval window and the lever-like action of the three bones.
- Transfer of sound vibration to fluid pressure waves. The cochlea contains fluid and an upper and a lower canal separated by a basilar membrane and cochlear duct (Fig. 36.8b). Vibrations of the oval window cause fluid pressure waves in both canals at nearly the same time.
- Mechanoreception by hair cells within the cochlea. The cochlear duct contains the organ of Corti, which has specialized hair cells with stereocilia supported by the basilar membrane. The stereocilia of mammalian hair cells form a “V” and project into a rigid tectorial membrane that does not move (Fig. 36.8c). The fluid vibrations within the cochlear canals induce motions of the basilar membrane relative to the tectorial membrane, bending the stereocilia of the hair cells back and forth in localized regions of the cochlea (Fig. 36.9). Bending of the stereocilia stimulates them to release excitatory neurotransmitters that cause postsynaptic neurons to fire action potentials. These are sensed as sound by neuronal networks in the auditory cortex, the area of the brain that processes sound.
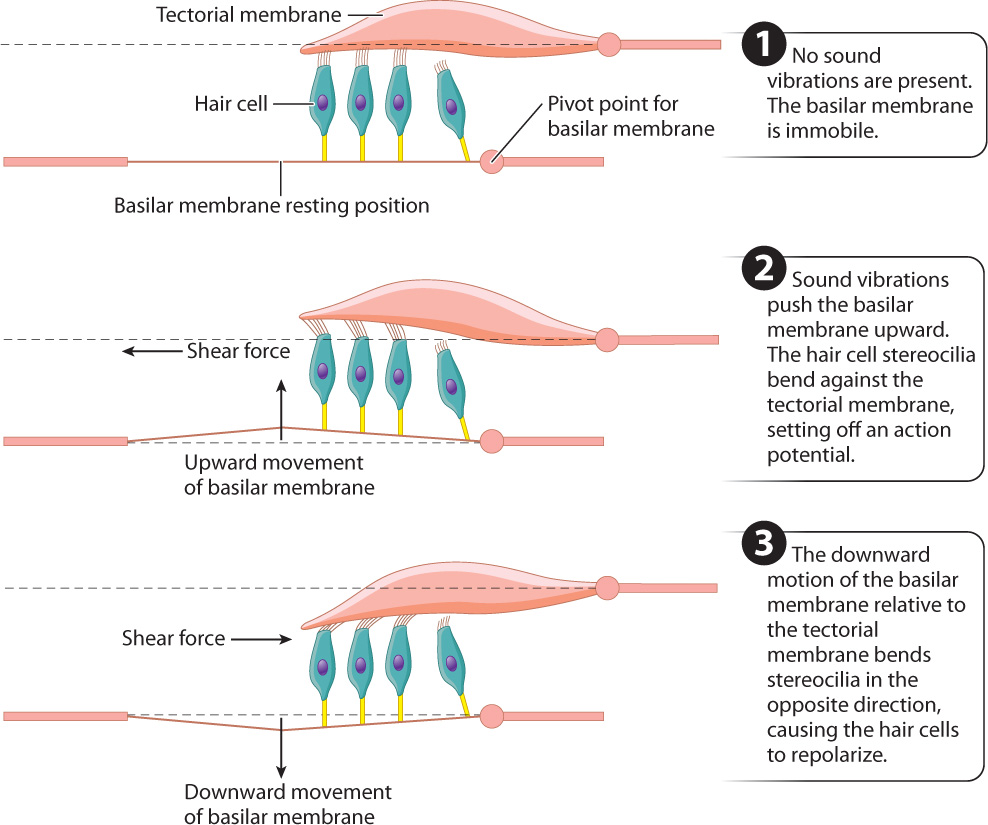
Sound is characterized by amplitude (loudness) and frequency (pitch). Louder sounds produce larger fluid vibrations that cause the hair cell stereocilia to bend more, increasing their release of excitatory neurotransmitters. The release of more neurotransmitters increases the firing rate of the postsynaptic cell. The firing rate indicates intensity, or in the case of hearing, loudness.
Many people can tell pure tones apart that differ by only a fraction of a percentage point in frequency. The ability to discriminate different sound frequencies is largely the result of differences in the mechanical properties of the basilar membrane along its length. At the apex, the basilar membrane is widest but thinnest and most flexible. At the base, the basilar membrane is narrow but thick and stiff. Thus, the basilar membrane is mechanically tuned to respond in different regions to different frequencies. Low-frequency vibrations excite hair cells in the apex of the basilar membrane, midrange-frequency vibrations excite hair cells in the middle region of the basilar membrane, and high-frequency vibrations excite hair cells closest to the base.
Sound amplitude and pitch, together with vestibular sensing of gravity and head motion, are transmitted by the vestibulocochlear nerve (another cranial nerve of the head) to the brain’s auditory cortex, discussed later in the chapter.
Case 7 Predator–Prey: A Game of Life and Death
36.3.3 How have sensory systems evolved in predators and prey?
Many insect-eating bats rely on echolocation to find and apprehend their flying prey (Fig. 36.10). As it flies, a bat emits short bursts of high-frequency sound. These sound pulses bounce off surrounding objects and are reflected back to the bat. The echoes are detected by the ears and processed by the bat’s brain to locate the prey. Bats typically increase their call rate as they approach the prey to locate it more accurately and judge its flight trajectory and its speed. The sharpness of an image is measured by its resolution, the smallest distance between two features or objects than can be perceived. Bats can resolve prey and other objects at less than 1 mm, an ability that exceeds that of the most sophisticated sonar developed by human engineers.

Together with mosquitoes and other flying insects, nocturnal moths are one of the bat’s primary food sources. In response to the evolution of echolocation by bats, several moths have evolved the ability to emit sounds that jam the bat’s sonar signal. These moths are more likely to escape capture. The evolution of sophisticated sensory systems in both groups of animals, and the brain organization that goes with it, is the result of a kind of evolutionary arms race. It illustrates how animal sensory systems and brains can achieve impressive detection and information processing performance that improves an animal’s chance of survival.