36.4 VISION
Electromagnetic receptors respond to electrical, magnetic, and light stimuli. Of these, light-detecting photoreceptors are the most common and diverse. Most animals sense light in their environment. Although animals have evolved different light-sensing organs, all rely on the same light-sensitive photopigment to convert light energy into a nervous signal. Their use of the same photopigment suggests a common evolutionary origin for the considerable diversity of animal eyes that dates from the Cambrian Period (approximately 500 million years ago), when diverse forms of multicellular animal life first evolved.
36.4.1 All animals use a similar photosensitive protein called opsin to detect light.
Most multicellular animals have cells called photoreceptors that respond to light. Animals with simple forms of photosensitivity can move toward or away from light sources. The photoreceptors of other animals are arranged to form eyes that produce an image. In some animals, detecting the motion of shapes and simple patterns of light is most important. Other animals, such as vertebrates and cephalopod mollusks like squid and octopus, perceive sharper images. Despite the diversity of light-sensitive organs and eyes that have evolved, all of them depend on a similar photosensitive protein known as opsin to convert the energy of light photons into electrical signals in the receptor cell.
Genes involved in the development of photoreceptors in flatworms are shared both with other invertebrates and with vertebrates, indicating that an early common ancestor of invertebrates and vertebrates first evolved the underlying biochemical machinery necessary for perceiving light. In these animals, a master regulatory gene, Pax6, is now known to function as a transcription factor regulating the development of the eye. In experiments performed by the Swiss developmental biologist Walter Gehring, Pax6 was recently found to regulate eye development in fruit flies and mice. Gehring and his group were able to insert the mouse Pax6 gene into fruit flies, leading to the formation of an additional small eye on the fly’s antenna (Chapter 20). Pax 6 has also been found expressed in flatworm photoreceptors. Remarkably, all light-sensing and image-formation systems therefore ultimately derive from the ancestor of the flatworm’s original photoreceptor.
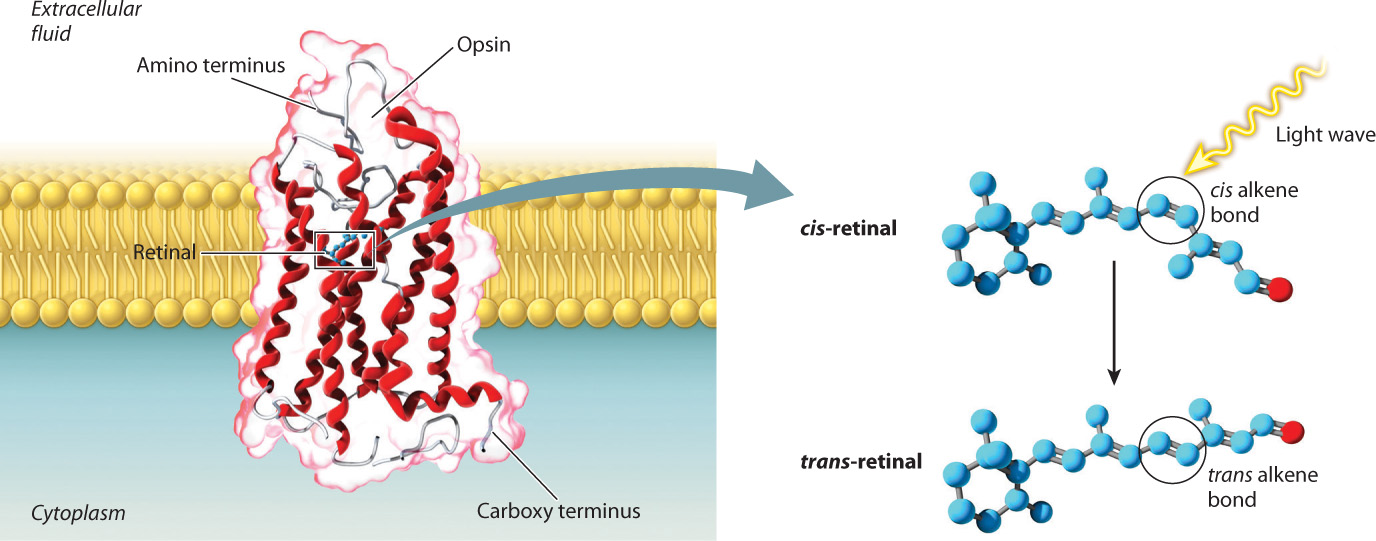
Rhodopsin is the specific transmembrane protein found in the photosensitive cells of vertebrates. This protein is covalently bound to retinal, a derivative of vitamin A that absorbs light (Fig. 36.11). Opsin molecules are arranged in cylindrical groups in the plasma membrane of most photosensitive cells. These cells are modified neurons with leaky Na+ channels. As a result, their resting membrane potential (in the dark) is less negative (about –35 mV) than that of other nerve cells. When retinal absorbs a light photon, it undergoes a conformational change from a cis to a trans configuration, and that conformational change causes Na+ channels to close. Without Na+ ions entering the cell, the cell membrane becomes hyperpolarized (rather than depolarized) and the cell’s neurotransmitter release is reduced. Photoreceptors themselves do not fire action potentials, but when stimulated by light energy they inhibit the firing rate of other neurons within the eye. The decrease in firing rate provides information about the intensity and pattern of light received.
36.4.2 Animals see the world through different types of eyes.
Although all eyes rely on similar opsin photopigments, the way the visual world is perceived—what it looks like to the organism—depends on the structure of the eye in which the receptors are embedded. Here, we look at three main types of eye structure shown in Fig. 36.12: eyecups, compound eyes, and single-lens eyes. Each has very different capabilities.
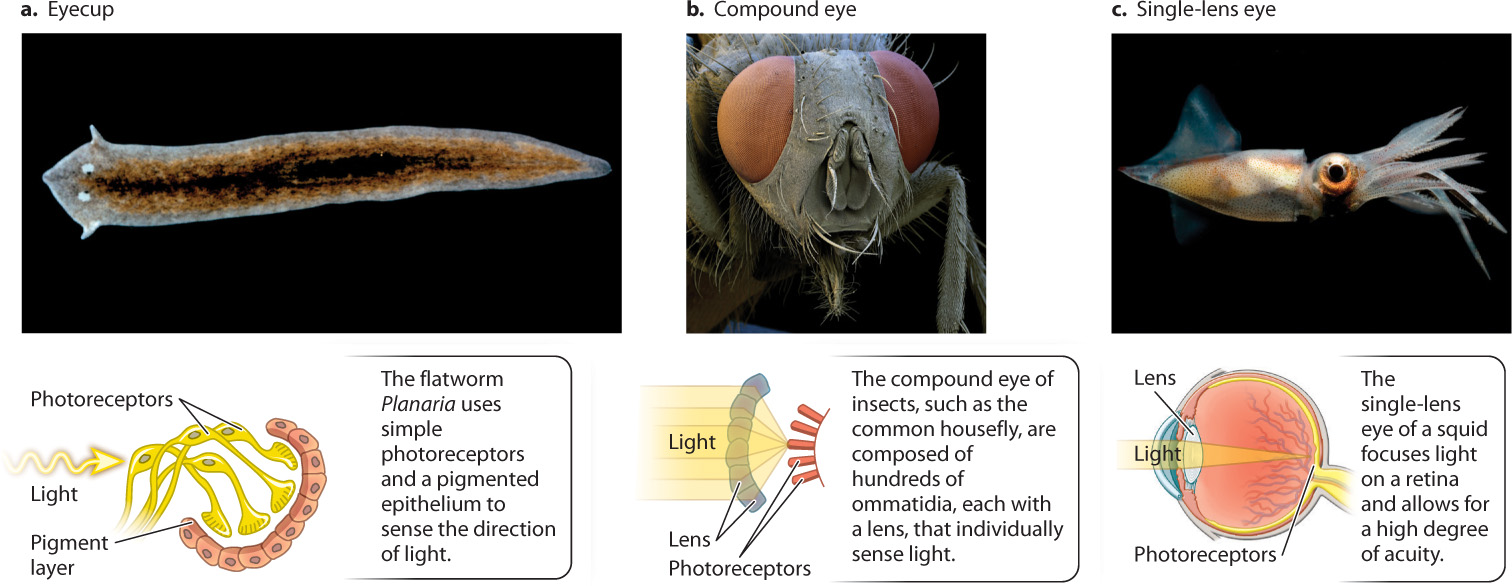
In flatworms, two simple eyecups on the dorsal, or back, surface of the head detect the direction and intensity of light sources (Fig. 36.12a). Each eyecup contains photoreceptors that point up and to the left or right. A pigmented epithelium blocks light from behind, so the photoreceptors receive light only from above and in front of the animal. In response to light, flatworms turn to move directly away from the light source, seeking a dark region that hides them from potential predators. To find a dark region, the flatworm compares the light intensity received by photoreceptors of the two eyecups, and then moves in the direction of lower light intensity. Many invertebrates use simple light-sensitive photoreceptors in a similar way.
Two major types of image-forming eye evolved in other invertebrates: compound eyes and single-lens eyes. Insects and crustaceans have compound eyes that consist of individual light-focusing elements called ommatidia (Fig. 36.12b). The number of ommatidia in the eye determines the resolution, or sharpness, of the image. The compound eyes of ants have only a few ommatidia, and those of fruit flies have about 800. In contrast, the eyes of dragonflies have 10,000 or more. Dragonflies are predators, and their high number of ommatidia is an adaptation for visually tracking their prey.
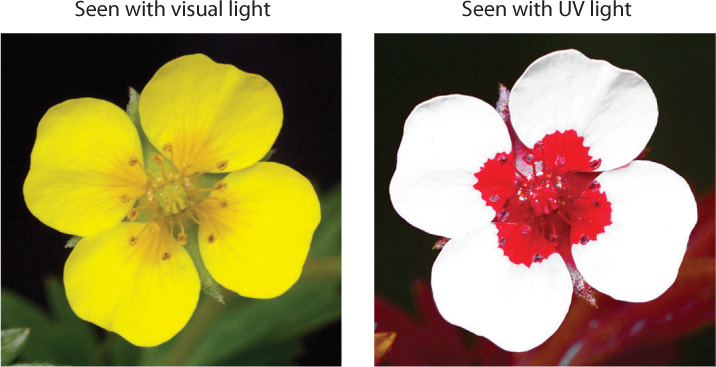
Within a single ommatidium, light is focused through a lens onto a central region formed from multiple overlapping photoreceptors. Each ommatidium is sensitive to a narrow angle of light (about 1° to 2°) in the animal’s visual field. Compound eyes of insects and crustaceans provide a mosaic image because individual light regions are sensed by separate ommatidia. It is likely that the image is sharpened within the brain, but the resolution of images produced by compound eyes is not nearly as good as those produced by single-lens eyes. Nevertheless, compound eyes are extremely good at detecting motion and rapid flashes of light, more than 300 per second compared to 50 per second for a human eye. Insect eyes also have good color vision, and many insects can perceive ultraviolet light. Many pollinating insects sense UV light to locate the flowers they prefer to visit for nectar (Fig. 36.13).
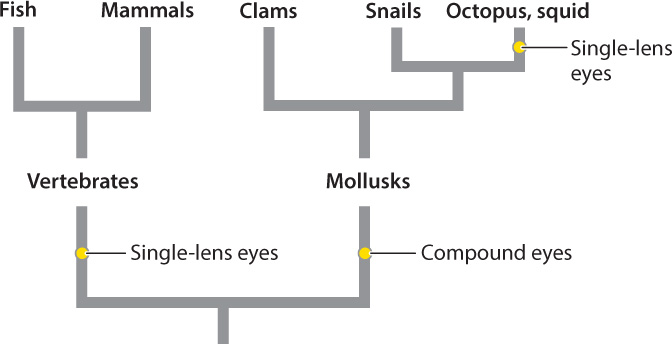
The single-lens eyes of vertebrates and cephalopod mollusks like squid and octopus work like a camera to produce a sharply defined image of the animal’s visual field (see Fig. 36.12c). In spite of their similar outward appearance, there is clear evidence that single-lens eyes evolved independently in vertebrates and in some mollusks (Fig. 36.14). Other invertebrates, such as some spiders and annelid worms, also evolved single-lens eyes independently. An advantage of single-lens eyes compared to compound eyes is that the single lens can focus light rays on a particular region of photoreceptors, improving both image quality and light sensitivity. As a result, animals with single-lens eyes can detect prey effectively by their motion and shape.
We focus much of our subsequent discussion on the human eye, but many of its organizational features and properties apply to vertebrates more generally.
36.4.3 The structure and function of the vertebrate eye underlie image processing.
Vertebrate eyes are generally protected within the eye socket of the skull. The anatomy of the eye is shown in Fig. 36.15. The eye is surrounded by the sclera, a tough, white outer layer. Below the sclera, a thinner layer, called the choroid, carries blood vessels to nourish the eye. Mucus secretions produced over the sclera keep the eye moist. The portion of the sclera in the front of the eye is transparent; this is the cornea. Light passes through the transparent cornea, enters the eye through an opening called the pupil, and then passes through a convex lens. Small muscles called ciliary muscles attach between the sclera and lens. Contraction or relaxation of these muscles adjusts the shape of the lens to focus light images.
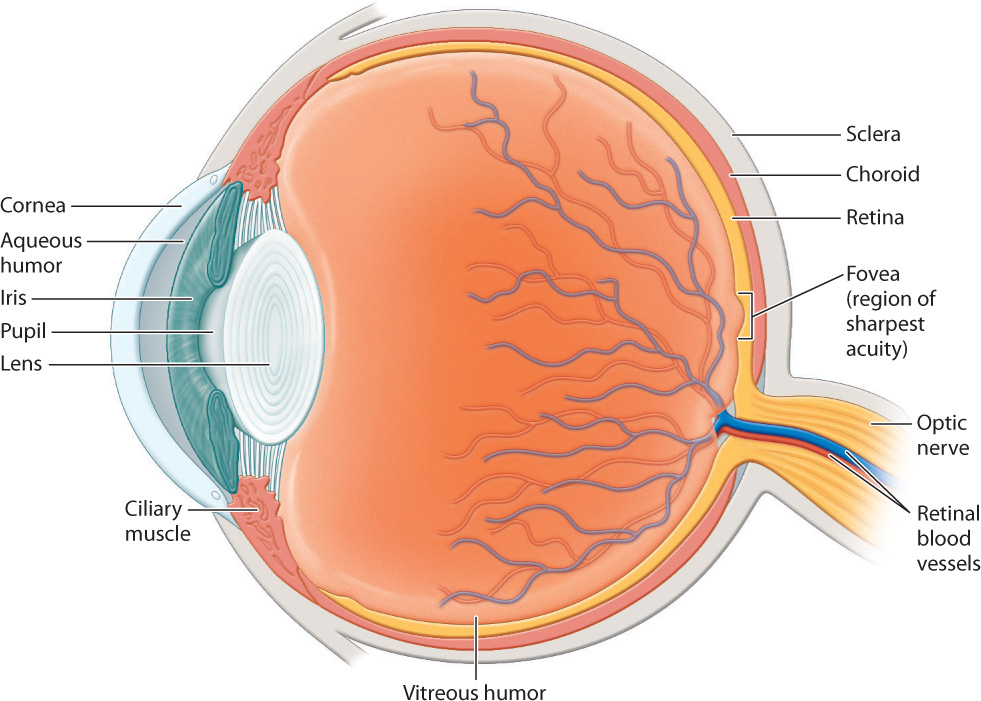
At the front of the eye, surrounding the pupil, the choroid forms a donut-shaped iris that opens and closes to adjust the amount of light that enters through the pupil, in the manner of the diaphragm of a camera. In bright light, the iris constricts to make the pupil small. In dim light, the iris dilates to allow in more light. The iris also gives the eye its color.
The focusing muscles and lens separate the eye into two regions. The interior region in front of the lens is filled with a clear watery liquid, the aqueous humor. This liquid is produced continuously and drained by small ducts at the base of the eye. Blockage of the ducts results in increased pressure within the eye that if sustained can cause glaucoma, which sometimes leads to blindness. The large cavity behind the lens is filled with a gel-like substance, the vitreous humor, that makes up most of the eye’s volume.
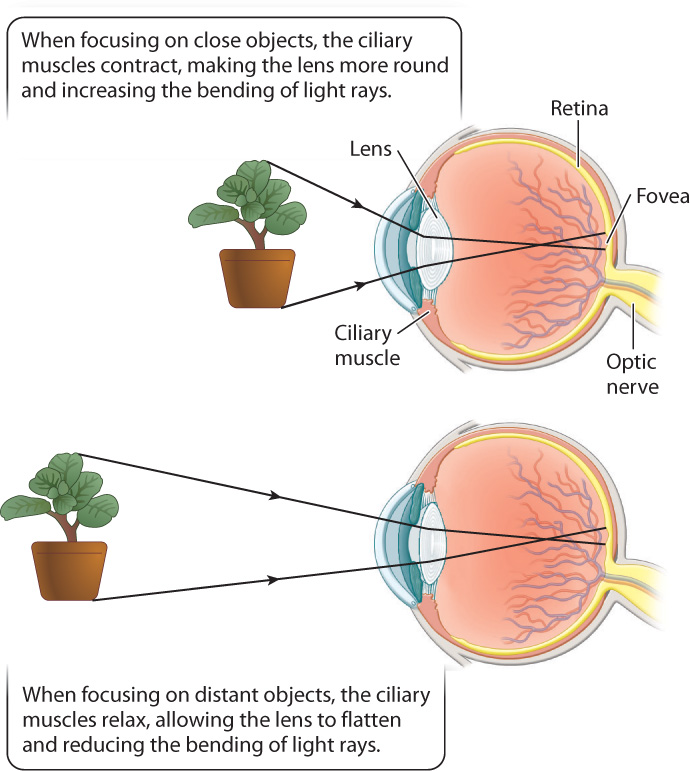
To form an image, the cornea and lens bend incoming light rays, focusing them on the retina (Fig. 36.15). In doing so, the light rays cross, inverting the image on the retina and changing up to down and left to right. The retina is a thin tissue located in the posterior of the eye. It contains the photoreceptors and other nerve cells that sense and initially process light stimuli. Humans, other mammals, and birds focus light on the retina by changing the shape of the lens (Fig. 36.16). When we focus on objects at close range, as when we read a book, the ciliary muscles contract, making the lens more rounded to bend the light rays more. When we focus on distant objects, the ciliary muscles relax, allowing the lens to flatten, reducing the diffraction, or bending, of light. In contrast, the eyes of octopus and squid focus by moving the lens back and forth within the eye, similar to a camera, rather than by changing its shape.
Why do so many animals have two eyes? One reason is that two eyes provide a wider field of vision. Close one eye and you will see the visual field of the other eye. With both eyes open, the visual field is wider than the visual field of either eye alone. You’ll also notice considerable overlap in the visual field of both eyes. This overlap exists in humans and other primates, as well as in other mammals, and makes possible binocular vision, the ability to combine images from both eyes to produce a single visual image. Binocular vision allows for depth and distance perception.
Prey animals, such as some birds, antelope, and rabbits, have eyes positioned more to the side, expanding their visual field up to 360°, but limiting or preventing binocular vision. Animals that require greater visual sharpness, such as predatory hawks, cats, and snakes, have eyes pointing forward, increasing their binocular field of view.
36.4.4 Color vision detects different wavelengths of light.
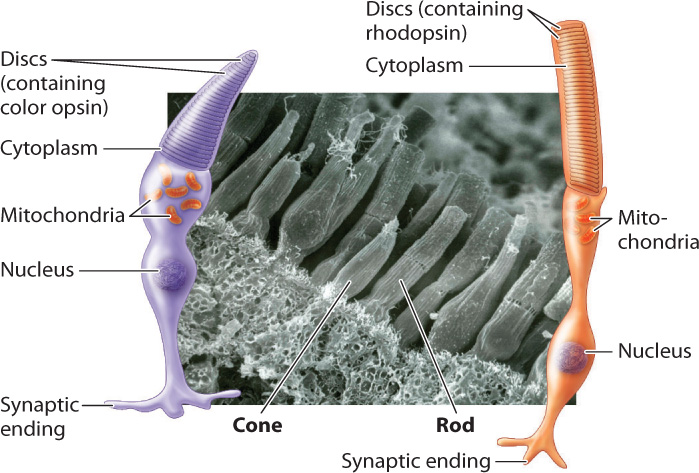
Color vision is crucial for many invertebrate and vertebrate animals. It is achieved by photoreceptor cells that contain visual pigments sensitive to different wavelengths of light (Fig. 36.17). Color-sensitive pigments are found within the cone cells of vertebrates. Cone cells are distinguished by their shape from the more numerous rod cells, which are sensitive to light but not color. Rod cells detect shades ranging from white to shades of gray and black. The human retina contains about 6 million cone cells and about 125 million rod cells. Because of their greater number and sensitivity to light, rod cells enable animals to see in low light. Together, rod and cone cells constitute approximately 70% of all sensory receptor cells in the human body, highlighting the importance of vision to perceive our environment.
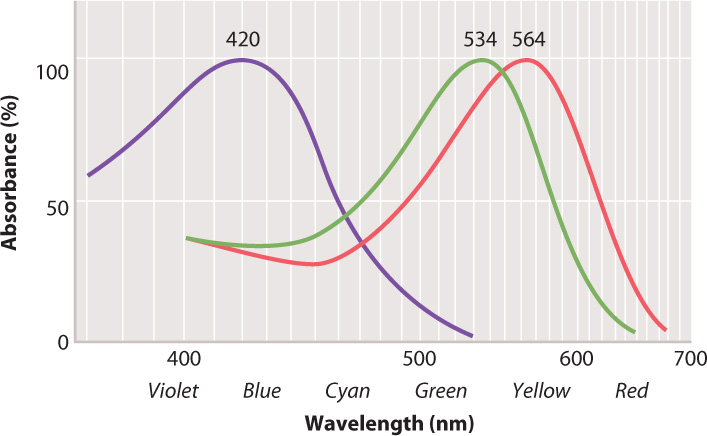
Vertebrate cone cells likely evolved from a rod cell precursor. Most vertebrates have cone cells with four photopigments, whereas the cone cells of most mammals have only two. It is likely that two photopigments were lost early in mammal evolution, when mammals were small, nocturnal, and burrowing. Old World primates, apes, and humans re-evolved trichromatic (that is, three-color) vision by means of gene duplication (some New World primates also regained trichromatic vision), likely in response to selection for better ability to locate fruit, a key part of their diet. Each human cone cell therefore has one of three photopigments, which absorb light at blue, green, or red wavelengths (Fig. 36.18). Stimulation of cone cells in varying combinations of these three photopigments allows humans and other primates to see a full range of color (violet to red). Fish, amphibians, reptiles, and birds also have good color vision, including for many the ability to see ultraviolet.
The low sensitivity of cone cells to light makes it hard to detect color at night. Cone cells are most concentrated within the fovea of the retina, the center of the visual field of most vertebrates (see Fig. 36.15). Cone cells provide the sharpest vision. Animals with particularly sharp vision, such as hawks and other birds of prey, have an extremely concentrated number of cone cells, approaching 1 million/mm2 (compared to about 150,000/mm2 in the human fovea). Birds of prey can see small prey on the ground from high in the air. Many birds also have eyes with two foveae, one projecting forward for binocular vision and one projecting to the side to enhance the sharpness of their side vision.
In contrast, rod cells are absent in the fovea but predominate in the periphery. Their positioning makes it easier to detect motion in the periphery, particularly at night. The eyes of many nocturnal predatory mammals, such as foxes and cats, contain mainly rod cells, enhancing nighttime vision. These animals also have a pigment within the retina that reflects light past the photoreceptors, enhancing the ability to sense light under low-light conditions. The reflection of light by this pigment creates the “eyeshine” that you see when a flashlight or a car’s headlights shines on a nocturnal predator’s eyes at night. Great white sharks are able to hunt at night because they also have a retinal reflective pigment.
36.4.5 Local sensory processing of light determines basic features of shape and movement.
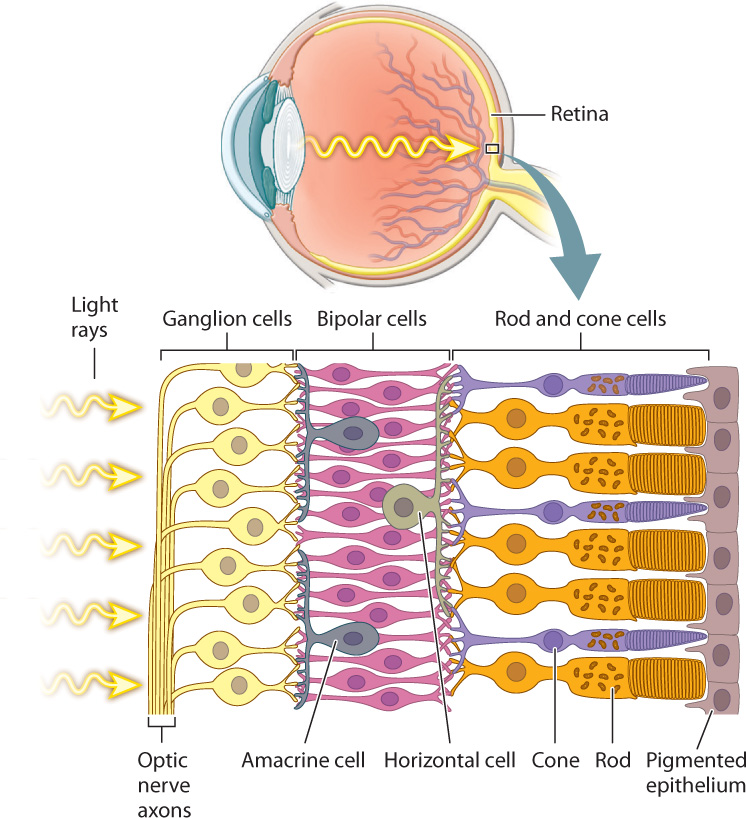
The rods and cones detect the intensity, color, and pattern of light entering the eye through the lens. However, it is their interaction with a highly ordered array of interneurons in the retina that begins the first steps of visual sensory processing (Fig. 36.19). The retina consists of five layers of neurons that form a signal-processing network just in front of a pigmented epithelium. The rods and cones are at the back of the retina, so that light must pass through several layers of neurons before reaching these light-sensitive cells. Because they are photoreceptors, rod and cone cells hyperpolarize in response to light but do not fire action potentials. They synapse on to bipolar cells, which also do not fire action potentials, but rather adjust their release of neurotransmitter in response to the input from multiple rod and cone cells. The bipolar cells, in turn, synapse on to ganglion cells located on the front of the retina. If activated, ganglion cells transmit action potentials by the optic nerve (a cranial nerve) to the visual cortex in the brain, the part of the brain that processes images. The optic nerve begins at the front of the retina and exits at the back, creating an area without light-sensitive cells: This is the blind spot. In octopus and squid, photoreceptors are in the front of the retina, not in the back as they are in vertebrates. As a result, octopus and squid don’t have a blind spot.
In addition to these three primary layers of neurons, two additional sets of nerve cells combine information laterally across the retina. Horizontal cells communicate between neighboring pairs of photoreceptors and bipolar cells, enhancing contrast through lateral inhibition to sharpen the image. Amacrine cells communicate between neighboring bipolar cells and ganglion cells, enhancing motion detection and adjusting for changes in illumination of the visual scene.
Photoreceptor cells, bipolar cells, ganglion cells, horizontal cells, and amacrine cells form a neural network that processes visual information before it is sent to the brain for further processing and interpretation. Visual signals from approximately 125 million photoreceptors converge to approximately 1 million ganglion cell neurons that communicate with the brain. Properties of this network were first studied in the mammalian retina by the American neurophysiologists Stephen Kuffler, David Hubel, and Torsten Wiesel in the 1950s and 1960s (Fig. 36.20). Hubel and Wiesel shared the 1981 Nobel Prize in Physiology or Medicine for their later work on how visual information is processed in the brain.
FIG. 36.20How does the retina process visual information?
BACKGROUND In the 1950s, the American neurophysiologist Stephen Kuffler was interested in understanding how the retina helps to process light information before it is sent to the brain. He focused on the activity of ganglion cells in the retina because they receive input from the photoreceptors and bipolar cells.
EXPERIMENT Kuffler stimulated different regions of the cat’s retina with localized points of light while recording the action potentials produced by ganglion cells.
RESULTS Kuffler found that there are two types of ganglion cell: on-center and off-center cells. On-center ganglion cells fire more action potentials when light shines on the center of the cell’s receptive field compared to the surrounding region, and off-center cells fire more when light is shown in the periphery and less on the center. These patterns are explained by lateral inhibition of input to the ganglion cells by the photoreceptors and bipolar neurons in the retina.
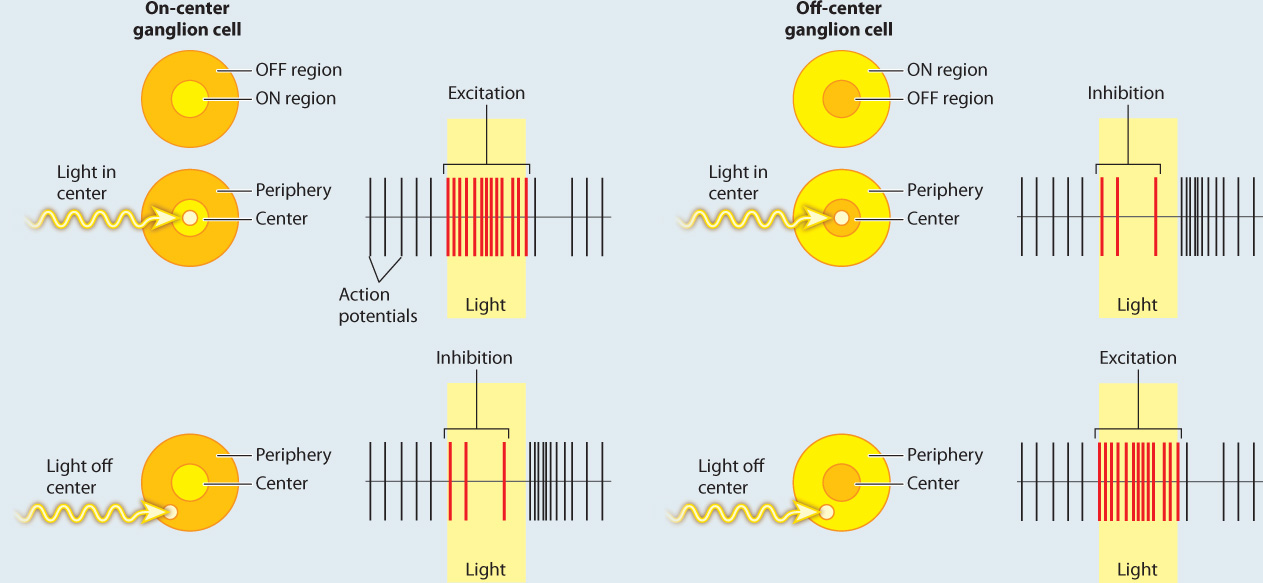
FOLLOW-UP WORK In the 1960s, Hubel and Wiesel found similar center–surround neural receptive fields, though with enhanced opposition, in part of the thalamus and in the visual cortex of the brain. Cells with these fields enable cats and other mammals to detect shapes of a given orientation moving through their visual field. Similar center–surround receptive fields have also been found in the somatosensory and auditory cortex, highlighting the use of lateral inhibition to enhance sensory acuity and edge detection. Other studies have found similar center–surround sensory processing in invertebrates and other vertebrates.
SOURCES Kuffler, S. W. 1953. “Discharge Patterns and Functional Organization of Mammalian Retina.” Journal of Neurophysiology 16 : 37–68; Hubel, D. H. 1963. “The Visual Cortex of the Brain.” Scientific American 209 : 54–62.
Quick Check 4
What is the primary role of rod cells, and what are the two roles of cone cells in the retina?