41.3 STRUCTURE AND FUNCTION OF THE MAMMALIAN KIDNEY
Mammals excrete urea dissolved in solution but can concentrate their urine more than their body fluids to minimize the loss of water, an adaptation to living on land. They concentrate their urine by generating concentrated (hypertonic) interstitial fluid deep in the kidney, through which the collecting ducts pass. Then, as the filtrate passes through the collecting ducts, water moves out of collecting ducts and into the interstitial fluid by osmosis, leaving concentrated urine in the collecting ducts. In this way, the urine is concentrated without the kidney ever actively transporting water itself. Instead, water flows passively by osmosis out of the collecting ducts. In addition, as we will see, nowhere along the length of the nephron are there large differences in osmotic pressure between the interstitial space and the renal tubules.

This process is quite efficient, and allows animals to thrive in diverse environments. For example, the giant kangaroo rat shown in Fig. 41.15, a desert mammal, reabsorbs so much water from the filtrate in its collecting ducts that it does not need to drink, obtaining enough water from seeds and cellular respiration. The giant kangaroo rat is thought to produce the most concentrated urine of all mammals. Marine mammals, such as whales, dolphins, and seals, also have exceptional abilities to concentrate their urine. They can remain at sea for long periods of time but cannot drink seawater for net water uptake. They, too, rely on metabolic water production in combination with the water in the food they eat to remain in water balance.
In this final section, we examine the mammalian kidney in more detail. The kidneys of birds share many features with those of mammals. However, these features arose independently in birds and mammals and are therefore the result of convergent evolution, presumably as adaptations for efficient processing of wastes in endothermic vertebrates with high metabolic rates (Chapter 40).
41.3.1 The mammalian kidney has an outer cortex and inner medulla.
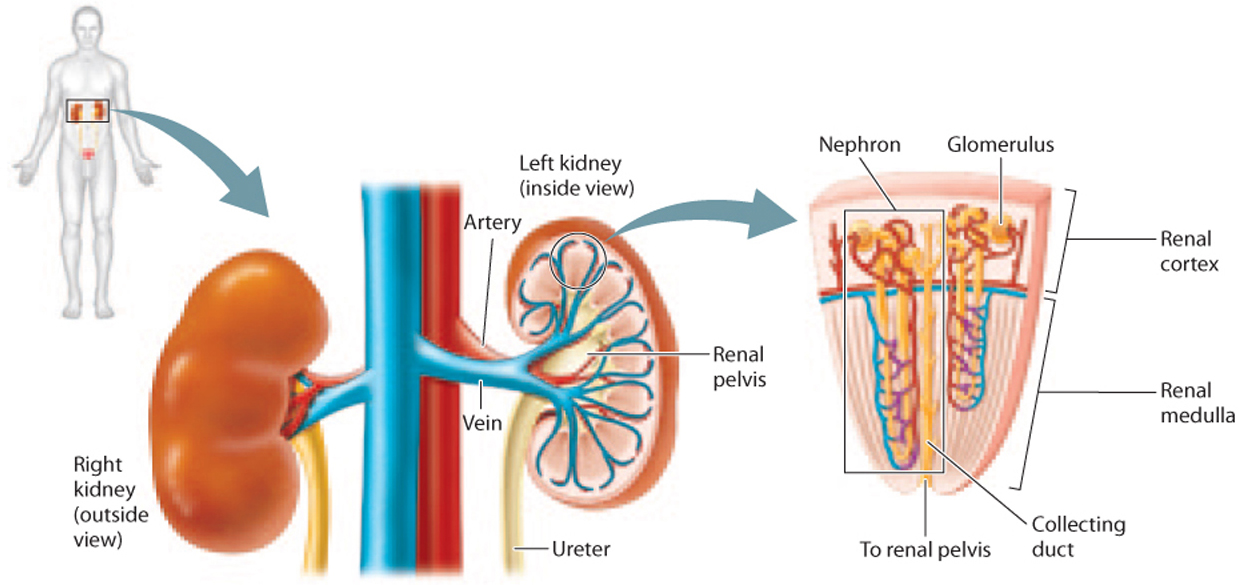
The paired kidneys of mammals are organized into two layers, an outer layer called the cortex and a deeper layer, surrounded by the cortex, called the medulla (Fig. 41.16). Nephrons are organized into wedge-shaped renal pyramids (Fig. 41.16). Each nephron’s glomerulus is located within the renal cortex or outer layer of the medulla, and feeds into a renal tubule that starts in the renal cortex before dipping into the medulla at the base of the renal pyramid and then looping back up to the cortex. The collecting ducts of the renal tubules also descend into the kidney’s base, emptying into the renal pelvis and draining through the ureter into the bladder.
About 20% of the blood circulated through the body with each beat of the heart passes through the kidneys for filtration. The kidneys very finely regulate the blood’s water and electrolyte content as they eliminate urea as a nitrogenous waste.
41.3.2 Glomerular filtration isolates wastes carried by the blood along with water and small solutes.
The glomerulus is a tuft of capillaries, with blood entering by an afferent (“toward”) arteriole and leaving by an efferent (“away”) arteriole (Fig. 41.17). The tuft of capillaries is encased in a membranous sac called Bowman’s capsule, and the space enclosed by the capsule is called Bowman’s space. Blood is filtered as it passes through the tuft of capillaries, the filtrate passing out of the capillaries, through Bowman’s capsule, and into Bowman’s space. Water, wastes, and solutes from the blood pass through the capillary wall into Bowman’s space before moving into the renal tubule.
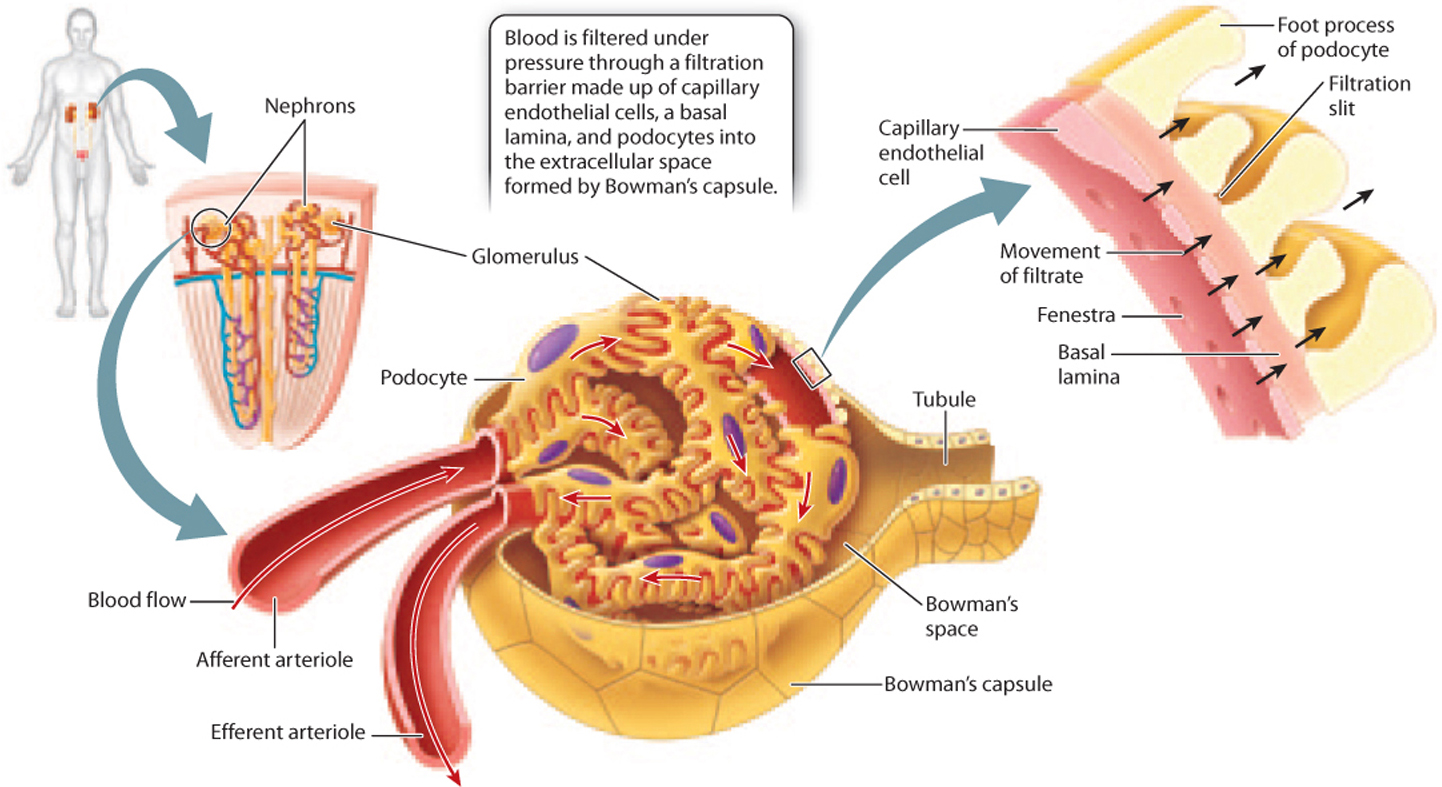
To allow filtration of blood, the capillaries of the glomerulus have a different structure from that of capillaries elsewhere in the body. The cells that line the capillaries, known as endothelial cells, have pores called fenestrae that allow fluid to pass through. The filtration barrier of the glomerulus is made up of three layers (Fig. 41.17): the endothelial cells; a basal lamina; and a layer of cells with footlike processes called podocytes, which loosely interlock to create slits in the cell layer. These three layers together create a filter that allows small molecules to pass through but blocks the passage of large proteins and cells, leaving them in the blood. As a result, wastes such as urea, as well as water and key solutes such as electrolytes, glucose, and amino acids, all end up in the filtrate. After passing into the Bowman’s space, the filtrate enters the renal tubule.
41.3.3 The proximal convoluted tubule reabsorbs solutes by active transport.
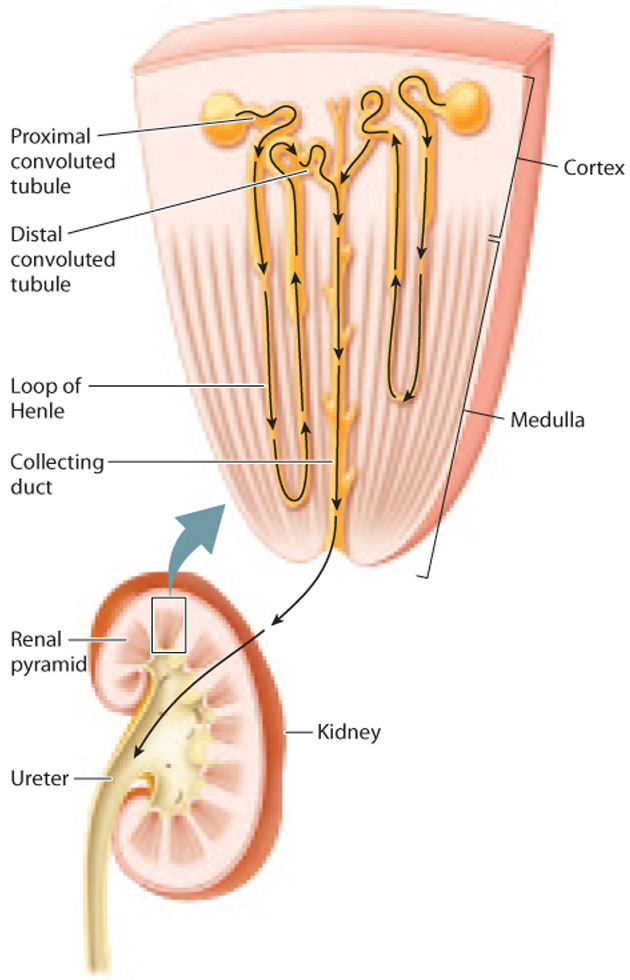
The renal tubule of the mammalian kidney is divided into three sections—a proximal convoluted tubule, a loop of Henle, and a distal convoluted tubule—as shown in Fig. 41.18. Although the renal tubule is one long tube, these three sections are specialized for different functions due to differences in permeability to different substances.
After passing through the filtration barrier and into Bowman’s space, the filtrate moves into the first portion of the renal tubule, the proximal convoluted tubule (Fig. 41.18). Here, electrolytes and other nutrients that the organism requires are reabsorbed into the blood. To maximize absorption, the proximal convoluted tubule has thick walls composed of epithelial cells with fingerlike projections called microvilli on their surfaces, similar in structure to those found in the small intestine (Chapter 40). The microvilli form a brush border along the lumen of the tubule, greatly increasing the cells’ surface area for solute and water reabsorption. The epithelial cells possess numerous mitochondria that produce the ATP needed to actively reabsorb solutes from the filtrate into the bloodstream.
The proximal tubule reabsorbs all the glucose and amino acids filtered by the glomerulus, as well as most sodium and chloride ions. Because the proximal tubule is permeable to water, water diffuses by osmosis in the same direction as the electrolytes and solutes. By the time the filtrate leaves the proximal convoluted tubule, about 75% of the water, along with most of the electrolytes and solutes, has been reabsorbed into the bloodstream.
41.3.4 The loop of Henle acts as a countercurrent multiplier to create a concentration gradient from the cortex to the medulla.
The proximal convoluted tubule connects to the loop of Henle, the second portion of the renal tubule (Fig. 41.18). (This structure was first described by the nineteenth-century German anatomist F. G. J. Henle.) Although many nephrons contain short loops of Henle that are confined to the renal cortex, some descend all the way into the medulla before looping back up to the cortex. These longer loops of Henle play an important role: They create a concentration gradient in the interstitial fluid from the cortex to the medulla, with the interstitial fluid of the cortex being less concentrated and the interstitial fluid of the medulla being more concentrated. This concentration gradient allows water passing through the downstream collecting duct to be reabsorbed, thus producing concentrated urine.
How is the gradient generated? The key to understanding how the loop of Henle creates a concentration gradient is to recognize that the two limbs of the loop of Henle run in parallel but in opposite directions, and they differ in their permeability to water and ability to actively transport electrolytes. Although the filtrate enters the descending limb before looping around to the ascending limb, it is easier to consider the ascending limb first.
The thick portion of the ascending limb of the loop of Henle is impermeable to water but actively transports electrolytes out of the filtrate (Fig. 41.19). As the filtrate moves up the thick ascending limb of the loop of Henle, electrolytes are actively transported out of it into the interstitial fluid surrounding the loop of Henle.
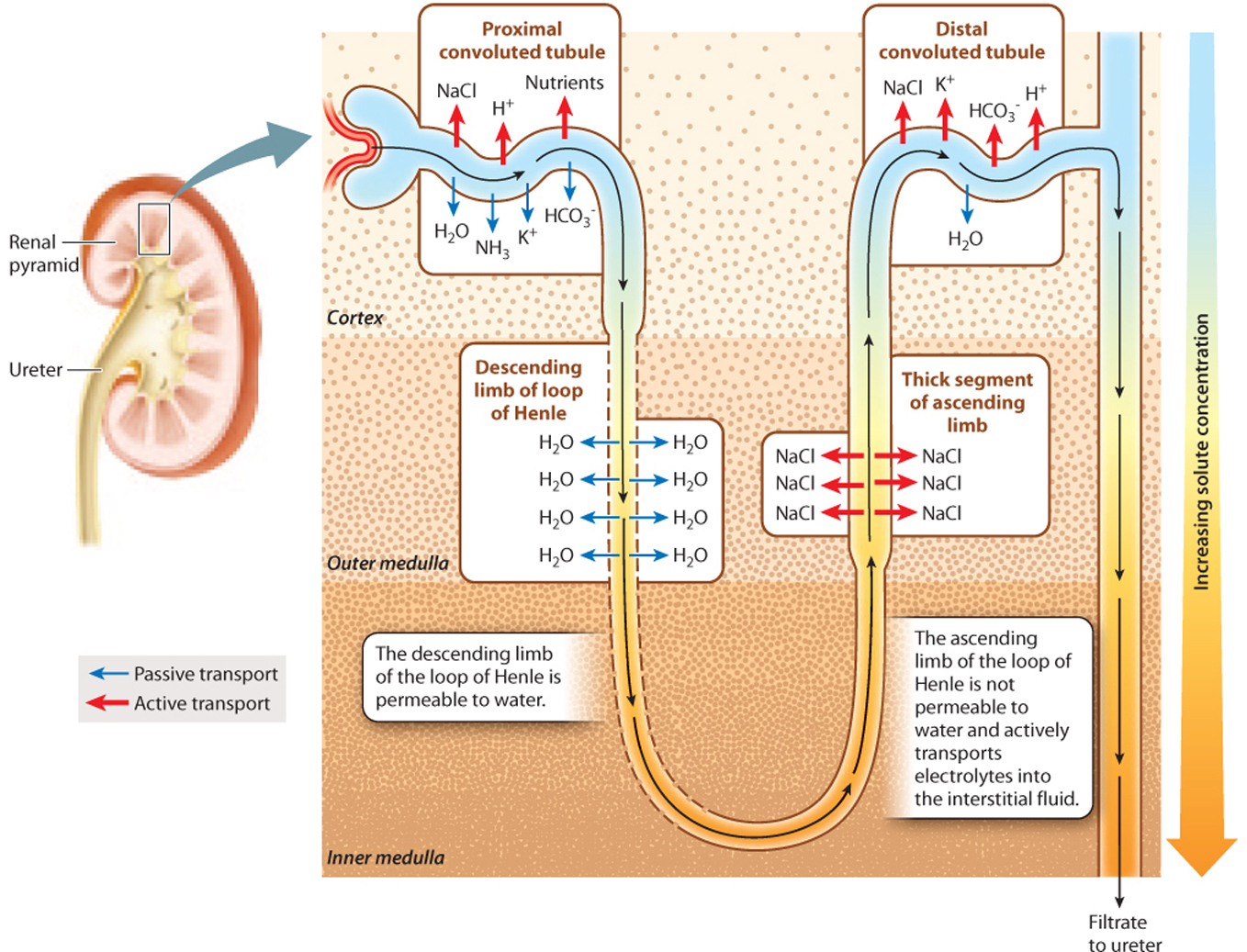
At the same time, the filtrate is moving from the descending limb to the ascending limb. As a result, the interstitial fluid becomes more concentrated, and the filtrate passing through the ascending limb becomes less concentrated. In contrast to the ascending limb, the descending limb is permeable to water. Therefore, water moves passively out of the descending limb, driven by osmosis. As water moves out of the limb, the filtrate becomes more and more concentrated, matching the concentration in the surrounding interstitial space. As electrolytes are pumped out of the thick ascending limb, the filtrate becomes less and less concentrated. Although the filtrate enters and leaves the loop of Henle at about the same concentration, the trip around the loop creates a concentration gradient in the interstitial fluid surrounding the loop of Henle, with fluid in the inner medulla much more concentrated than that in the outer cortex.
At the base of the loop, the filtrate still contains some electrolytes, but the main solute is urea because electrolytes were actively reabsorbed into the bloodstream from the proximal tubule and because the descending portion of the tubule is impermeable to urea. Then, as additional electrolytes are actively transported out of the thick ascending limb, the concentration of the filtrate (now mainly urea) decreases because the tubule is impermeable to water.
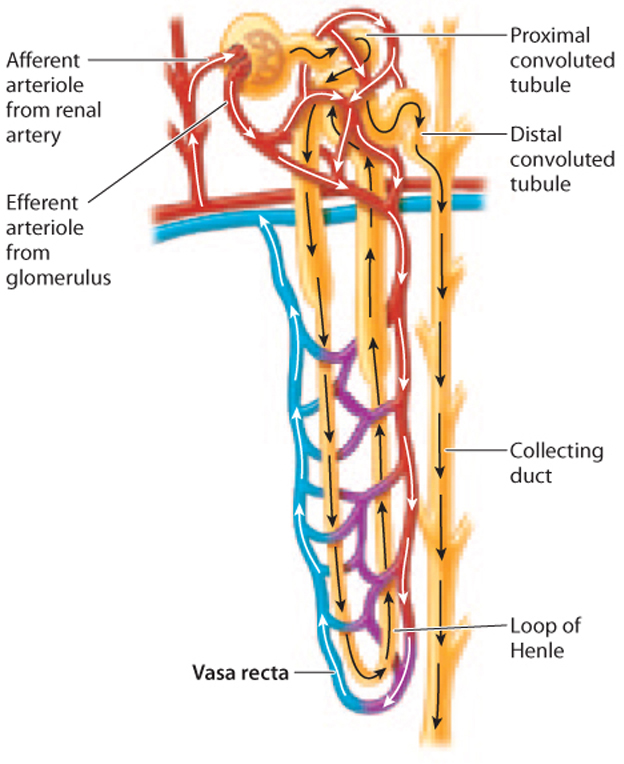
In Chapter 39, we saw how the movement of water and blood in opposite directions in fish gills provides an efficient way to extract oxygen from the water and move it into the blood. This mechanism, called countercurrent exchange, is an efficient mechanism for two fluids to exchange properties, in this case, the amount of oxygen in each of the fluids. In the loop of Henle, active transport of electrolytes creates a concentration gradient, which is then multiplied because the descending and ascending limbs move in parallel but in opposite directions. This system is therefore known as a countercurrent multiplier. While countercurrent exchangers maintain a concentration gradient, countercurrent multipliers generate them.
There is one more important point to consider. Typically, a high concentration of solutes in the interstitial fluid would be quickly lost due to water flowing out of blood vessels by osmosis. To avoid dissipating the concentration gradient, the blood vessels in the kidneys are also arranged in a particular fashion. The blood vessels, collectively called the vasa recta, have descending and ascending vessels arranged in a countercurrent organization, just like the loops of Henle (Fig. 41.20). The blood within these vessels flows down into the medulla in one arm and back up through the cortex in the other. This arrangement allows the concentration gradient to be maintained. Blood flowing from the cortex to the base of the kidney encounters increasingly concentrated interstitial fluid, allowing the passive diffusion of water out of the blood. However, the descending vessels pass in proximity to ascending vessels, which reabsorb the water. In effect, water passes straight from one arm of the vasa recta to the other, meaning the interstitial fluid and the bloodstream end up with no net gain or loss of water. As a result, the concentration gradient from the cortex to the medulla is maintained.
The generation of a concentration gradient by a countercurrent multiplier in the loop of Henle is a key step in the production of urine that is more concentrated than the blood. It was first proposed by the Swiss chemist Werner Kuhn in the 1950s. Although initially met with skepticism, subsequent experimental work confirmed predictions of his hypothesis (Fig. 41.21).
Quick Check 4
The filtrate is dilute at the start and end of the loop of Henle. What, then, is the function of the loop of Henle?
FIG. 41.21How does the mammalian kidney produce concentrated urine?
BACKGROUND The mammalian kidney produces urine that is more concentrated than the blood. How it concentrates urine was one of the most perplexing problems in renal physiology.
HYPOTHESIS One hypothesis to explain how the kidney concentrates urine is that the kidney actively transports water out of the collecting ducts as the filtrate leaves the kidney. Another, first suggested by the Swiss chemist Werner Kuhn in the early 1950s, is that the loop of Henle creates a concentration gradient from the cortex to the medulla by a countercurrent multiplier mechanism. According to this model, the solute concentration is low in the cortex and high in the medulla, so water leaves the collecting ducts passively as it passes from the cortex to the medulla.
PREDICTION A prediction of Kuhn’s hypothesis is that the solute concentration of fluid taken at the same level from the medulla, loop of Henle, and collecting ducts should be similar to one another, and higher than that of the blood. If, instead, water is simply pumped out of the collecting ducts, then the fluid in the collecting ducts should be more concentrated than that in the loop of Henle.
EXPERIMENT American physiologists Carl W. Gottschalk and Margaret Mylle tested Kuhn’s hypothesis. They used innovative micropuncture techniques in hamsters to measure the solute concentration of fluid taken from the bend of the loop of Henle and the collecting ducts at the same level.
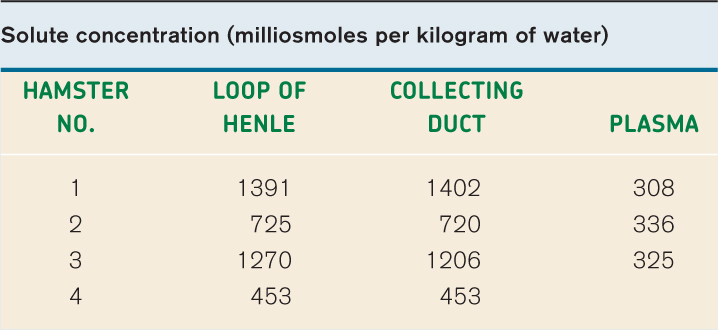
RESULTS Their results, shown in the table, are consistent with the predictions of Kuhn’s hypothesis. That is, the solute concentrations of the fluid in the loop of Henle and in the collecting ducts are similar to each other and higher than that of the blood.
CONCLUSION These results are consistent with a model in which concentrated urine is produced by first generating a concentration gradient between the cortex and the medulla, and then allowing water to move out of the collecting ducts by osmosis to produce concentrated urine.
FOLLOW-UP WORK Similar measurements in other areas of the kidney and in other mammals and birds supported the model.
SOURCES Gottschalk, C. W., and M. Mylle. 1958. “Evidence That the Mammalian Nephron Functions as a Countercurrent Multiplier System.” Science 128:594; Gottschalk, C. W., and M. Mylle. 1959. “Micropuncture Study of the mammalian urinary concentrating mechanism: evidence for the countercurrent hypothesis.” American Journal of Physiology 196 (4):927–936.
41.3.5 The distal convoluted tubule secretes additional wastes.
The filtrate entering the distal convoluted tubule, which is located within the cortex, is hypotonic relative to the interstitial space, having lost nearly all of its electrolytes. As a result, urea remains the principal solute. Other wastes from the bloodstream are also actively secreted into the distal convoluted tubule.
At this point, then, the filtrate is dilute, and the main solutes are urea and other wastes. The dilute filtrate enters the collecting ducts, which drain into the renal pelvis at the base of the kidney, passing from the cortex to the medulla. The concentration gradient established by the loop of Henle from the cortex to the medulla allows water to be reabsorbed as needed depending on the water levels of the organism, as we will see next.
41.3.6 The final concentration of urine is determined in the collecting ducts and is under hormonal control.
The final segment of the nephron is the collecting duct. At this point, the key processes of filtration, reabsorption, and secretion have occurred. The filtrate is dilute but contains wastes and water. The collecting duct is the site where water levels are adjusted to meet the osmoregulatory needs of the organism and therefore maintain homeostasis. The concentration gradient from cortex to medulla established by the loop of Henle allows the collecting ducts to regulate water levels.
The permeability of the collecting ducts to water determines how dilute or concentrated the urine is. The water permeability of the collecting ducts is controlled by the peptide hormone antidiuretic hormone (ADH), also called vasopressin, which is secreted by the posterior pituitary gland (Chapter 38). In the presence of ADH, the collecting duct walls become permeable to water (Fig. 41.22). This change in permeability occurs because aquaporins insert in the membrane of cells of the collecting duct. The aquaporins allow water in the collecting duct to diffuse into the interstitial fluid, thus making the urine in the tubule more concentrated. When a person is dehydrated, the urine leaving the collecting ducts can become as concentrated as the interstitial space at the base of the kidney.
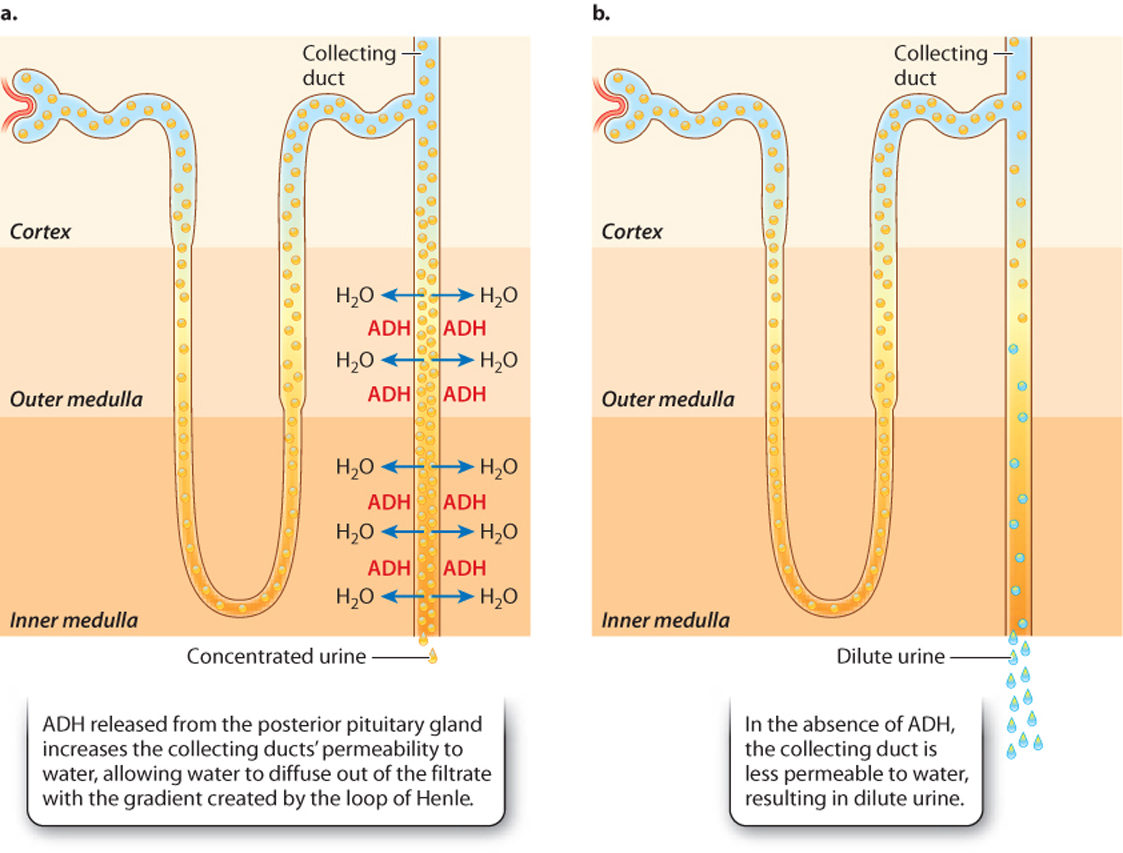
In the absence of ADH, the collecting duct walls are impermeable to water, preventing the water from leaving. This produces dilute urine. Some diuretic substances ("diuretic" is from the Greek word meaning "to flow through"), such as alcohol, promote the dilution of uring by interfering with ADH production and therefore making the collecting duct walls more impermeable to water. If hypotonic urine is produced, there is net water loss. Consequently, diuretic drinks often cause dehydration.
The regulation of water and solute reabsorption by the kidney is remarkably precise. The kidneys of a human filter 180 liters (L) of blood each day (125 ml/min). The total blood volume of a human is about 5 L, which means that the blood is filtered about 60 times per day. Urine production in humans averages 0.5 L per day, which means that under normal conditions, the kidneys reabsorb 99.9% of the water entering the renal tubules through glomerular filtration. In addition, the kidneys reabsorb all the glucose and amino acids that were filtered out of the blood in the glomerulus, together with 99.9% of the electrolytes in the blood. Human kidneys achieve this efficient transport of key ions, solutes, and water because of the renal tubules’ extensive microvilli and the large number of mitochondria and membrane transporters in their epithelial cells.
The exceptional ability of the kidneys to filter and reabsorb key ions, solutes, and water also shows why kidney damage can have such serious consequences. People with kidney disease require that their blood be filtered by dialysis treatment as frequently as three times per week, each treatment lasting several hours. Otherwise, toxic wastes would build up to dangerous levels in the body. Renal failure resulting from kidney disease remains a substantial health problem in the world today, costing more than $34 billion in the United States alone.
41.3.7 The kidneys help regulate blood pressure and blood volume.
Because the kidneys receive a large fraction of the blood leaving the heart, they are well suited for monitoring changes in blood pressure and maintaining it at relatively constant levels, another example of homeostasis. Specialized cells of the efferent arteriole leaving the glomerulus of each nephron form the juxtaglomerular apparatus (Fig. 41.23). In response to a drop in blood pressure, these cells secrete the hormone renin into the bloodstream. Renin stimulates epithelial cells of the lung to release an enzyme that converts an inactive hormone (angiotensinogen) in the bloodstream into its active form, angiotensin II. Angiotensin II acts on the smooth muscles of arterioles throughout the body, causing them to constrict, thus increasing blood pressure and directing more blood back to the heart.
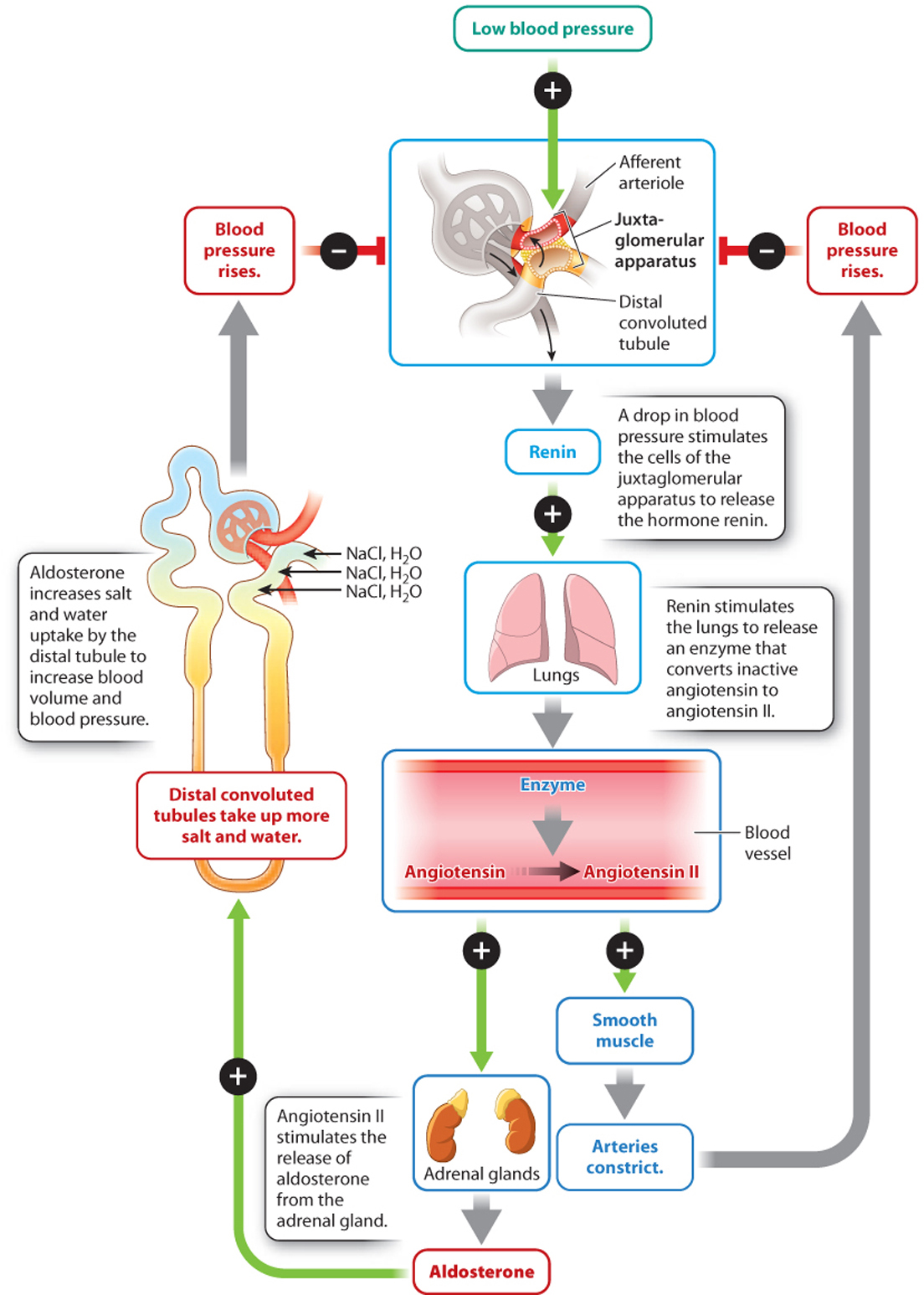
Angiotensin II also stimulates the release of aldosterone, a hormone produced by the adrenal glands that stimulates the distal convoluted tubule and collecting ducts to take up more salt and water. Increased salt and water uptake in turn leads to increased blood volume and therefore blood pressure. Thus, the kidneys participate in the control of blood pressure that is also monitored by baroreceptors (specialized mechanoreceptors that sense blood pressure) in the aorta and carotid bodies. These communicate with the autonomic nervous system to control heart rate and contractile force, as discussed in Chapter 39.
The kidneys, therefore, play many roles. In addition to the elimination of wastes and other toxic compounds, they serve important homeostatic functions, including water and electrolyte balance and the regulation of blood volume and blood pressure.