43.1 INNATE IMMUNITY
The immune system consists of two parts that work in different ways and interact with each other to protect against infection. The first, called innate, or natural, immunity, provides protection in a nonspecific manner against all kinds of infection. This form of immunity is present in plants, fungi, and animals and is an evolutionarily early form of immunity. It is considered innate because it does not depend on exposure to a pathogen. By contrast, the second form of immunity, called adaptive, or acquired, immunity, is specific to a given pathogen. This form of immunity “remembers” past infections, and subsequent encounters with the same pathogen generate a stronger response from the host. In other words, the immune system adapts over time, and immunity is acquired after an initial exposure. This form of immunity evolved later than the innate form and is unique to vertebrates. Table 43.1 summarizes the major differences between these two forms of immunity.
| Features of the Innate and Adaptive Immune Systems | |||||
|---|---|---|---|---|---|
| INNATE IMMUNE SYSTEM | ADAPTIVE IMMUNE SYSTEM | ||||
| Targets | Diverse pathogens | Diverse pathogens | |||
| Ability to distinguish self from non-self? | Yes | Yes | |||
| Specificity? | No | Yes | |||
| Memory? | No | Yes | |||
| Organisms | All organisms | Vertebrates | |||
We begin by considering forms of defense in the innate immune system. These include tissues, such as the skin, that act as a barrier to infection, cells that act as sentries against pathogens, and proteins and other molecules circulating in the blood that signal the presence of infection. All these defenses are nonspecific, act immediately, and do not depend on past exposure to elicit a response.
43.1.1 The skin and mucous membranes provide the first line of defense against infection.
Organisms have a barrier that surrounds them, like the walls of Troy, which can at times be breached. Ordinarily, the barrier around organisms, among its other functions, protects against infection. Examples include the cell wall of bacteria, the bark of trees, the cuticle of leaves, the exoskeleton of insects, the scales of fish, the shells of eggs, and the skin of mammals (Fig. 43.1). These structures act as physical barriers against infection.

To understand the importance of the barrier, consider what happens when it is breached. Burn victims who lose the outer layer of their skin are particularly vulnerable to infection. The bite of a dog or the sting of an insect can seed viruses, bacteria, or parasites directly into the bloodstream, bypassing the protective layer of skin. Even a small puncture wound can provide entry for the bacterium Clostridium tetani, the cause of tetanus.
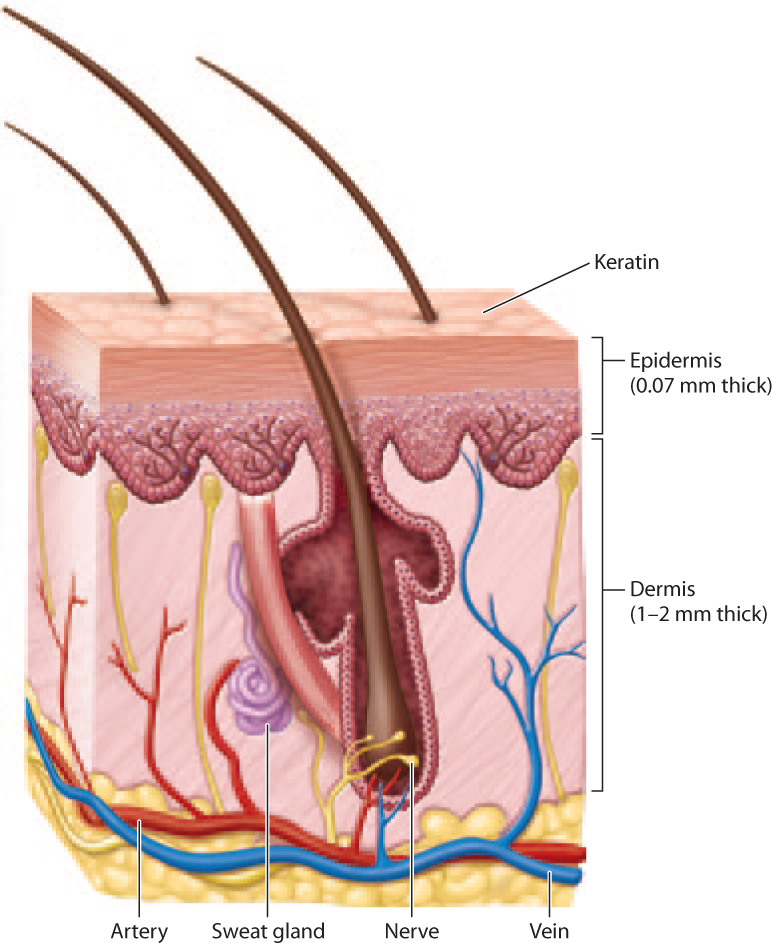
In humans, this physical barrier is the skin (Fig. 43.2). It has two layers, the outer epidermis and the inner dermis (Chapter 10). The epidermis consists of several layers of cells covered with the protein keratin. The dermis consists of connective tissue, hair follicles, blood and lymphatic vessels, and glands. Together, these two layers provide a physical barrier against microorganisms.
Any surface that is in contact with the external environment is a potential entry site for pathogens. Like the skin, the mucous membranes of the respiratory, gastrointestinal, and genitourinary tracts act as physical barriers. However, they have additional protective mechanisms. For example, parts of the respiratory tract have cilia, hairlike projections that sweep mucus along with trapped particles and microorganisms out of the body. Saliva and tears contain enzymes that help to protect against bacteria. The stomach, with its low pH and enzymes that break down food, is inhospitable to many microorganisms.
In addition to acting as a physical and chemical barrier, the skin and mucous membranes provide a home to many nonpathogenic microorganisms. By one estimate, of the 100 trillion cells in the human body, only 10% are human—the rest are mostly bacteria. These microorganisms in some cases provide protection against harmful pathogens by competing with them for food and space. They may also maintain health in other ways, such as by aiding digestion. In this way, we live in a mutualistic relationship with these microorganisms (Case 5: The Human Microbiome). The Human Microbiome Project, sponsored by the National Institutes of Health, is an effort to describe and understand the ecology of these microorganisms.
Quick Check 1
Certain diseases, as well as cigarette smoke, can damage cilia lining the respiratory tract. What effects would you expect from damage to respiratory cilia?
43.1.2 Some cells act broadly against diverse pathogens.
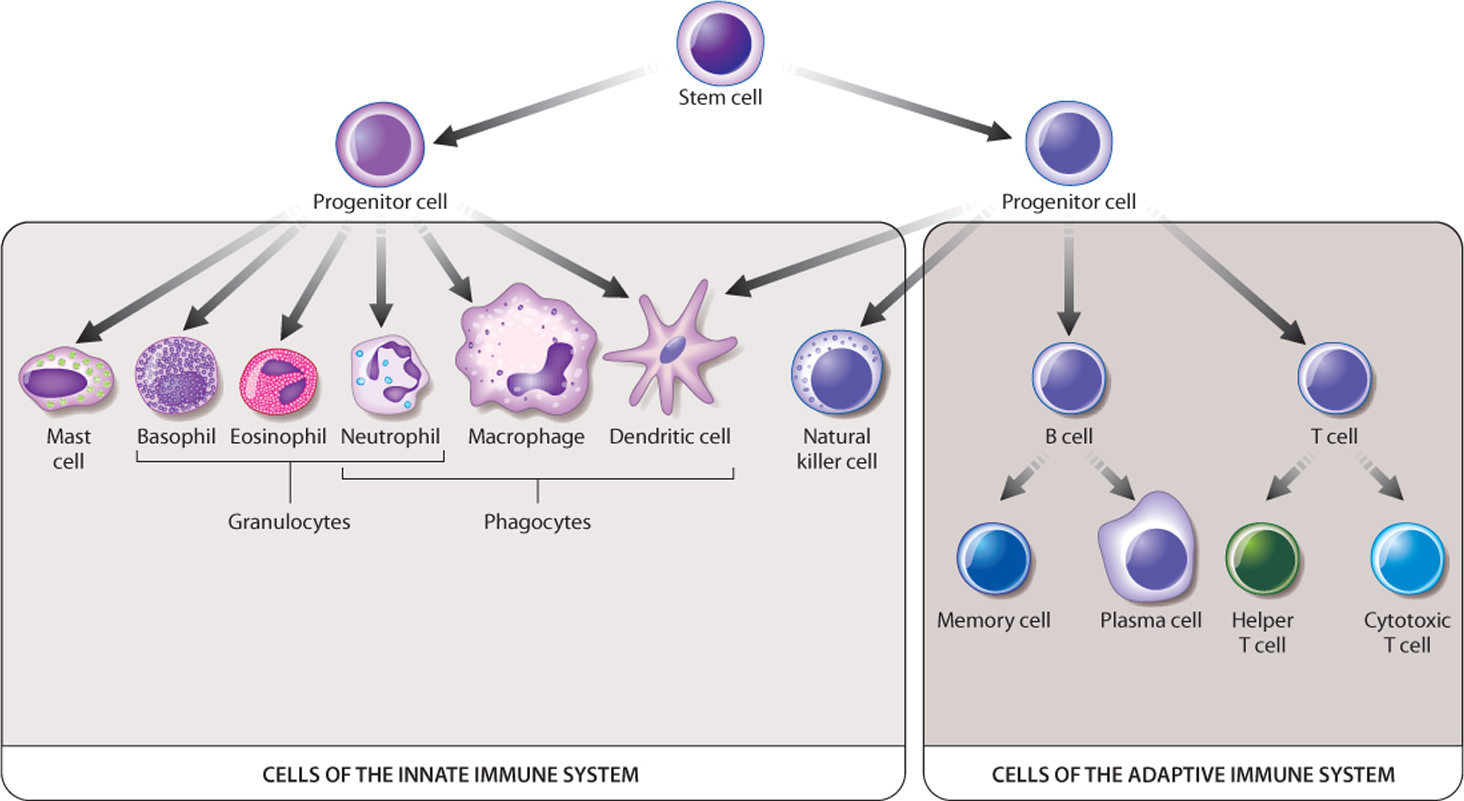
The innate immune system has numerous cells in addition to those in the skin and mucous membranes, found in different locations in the body and having different mechanisms of action. These cells are white blood cells, or leukocytes, and they arise by differentiation from stem cells in the bone marrow (Fig. 43.3). Many of these cells have important roles in the adaptive immune response as well. Here, we focus on their role in innate immunity.
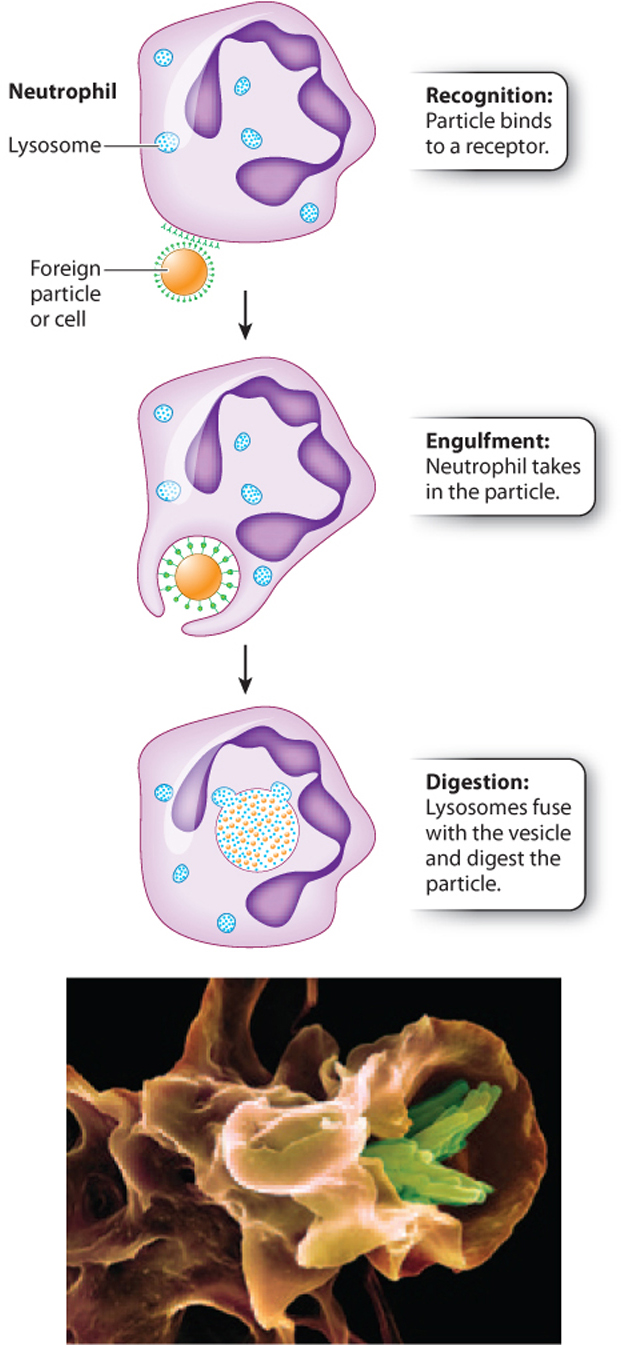
Phagocytes (literally, “eating cells”) are immune cells that engulf and destroy foreign cells or particles. The engulfing of a cell or particle by another cell is called phagocytosis. The Russian microbiologist Ilya Mechnikov first described this remarkable process, for which he shared the Nobel Prize in Physiology or Medicine with the German scientist Paul Ehrlich in 1908.
The process begins when a phagocyte encounters a cell or particle that it recognizes as foreign and binds to it. The phagocyte then extends its plasma membrane completely around the cell or particle until it is within a separate compartment inside the phagocyte (Fig. 43.4). In some cases, the compartment merges with a lysosome, and enzymes within the lysosome digest the foreign cell or particle. Phagocytosis can also trigger a respiratory burst, a process that generates reactive oxygen species, such as superoxide radicals and hydrogen peroxide, and reactive nitrogen species, such as nitrogen oxide and nitrogen dioxide. These molecules have antimicrobial properties that directly damage pathogens.
There are three major types of phagocytic cell: macrophages, dendritic cells, and neutrophils (see Fig. 43.3). In each case, their names give clues to their morphology. Macrophages are large cells that patrol the body (macro means “large”). Dendritic cells have long cellular projections reminiscent of the dendrites of neurons (Chapter 35), but they are not part of the nervous system. These cells are typically part of the natural defenses found in the skin and mucous membranes. Neutrophils are members of a group of cells called granulocytes because of the presence of granules in their cytoplasm. The granules of neutrophils take up both acidic and basic dyes, so their staining pattern is considered neutral. Neutrophils are very abundant in the blood and are often the first cells to respond to infection.
The innate immune system contains several other cells in addition to phagocytic cells (see Fig. 43.3). Two types of granulocyte, eosinophils and basophils, defend against parasitic infections but also contribute to allergies. Mast cells release histamine, an important contributor to allergic reactions and inflammation, discussed below. Finally, natural killer cells do not recognize foreign cells, but instead recognize and kill host cells that are infected by a virus or have become cancerous or otherwise abnormal.
43.1.3 Phagocytes recognize foreign molecules and send signals to other cells.
Phagocytes attack foreign cells that they encounter but leave host cells alone. How is a phagocyte able to distinguish a foreign cell from a host cell? All cells have an array of proteins and other molecules on their surfaces. Phagocytes detect specific surface molecules that are present on pathogens but not on the body’s own cells.
Toll-like receptors (TLRs) play a critical role here. TLRs are a family of transmembrane receptors present on phagocytes that recognize and bind to molecules on the surface of microorganisms. As a result, they provide one of the earliest signals that an infection is present. TLRs detect a wide range of microorganisms by recognizing evolutionarily conserved surface molecules shared by many microorganisms. For example, one type of TLR recognizes a glycolipid on the cell wall of certain bacteria. Another recognizes a protein that is part of the bacterial flagella.
Binding of the TLR to surface molecules on the pathogen is a signal to the phagocyte to engulf and destroy its target. Phagocytes also contribute to the immune response in another way. Following binding of a foreign molecule to the TLR, they send a message to the rest of the immune system as well. Phagocytes release chemical messengers called cytokines that recruit other immune cells to the site of injury or infection. Cytokines, like hormones, provide long-distance communication between cells (Chapters 9 and 38).
43.1.4 Inflammation is a coordinated response to tissue injury.
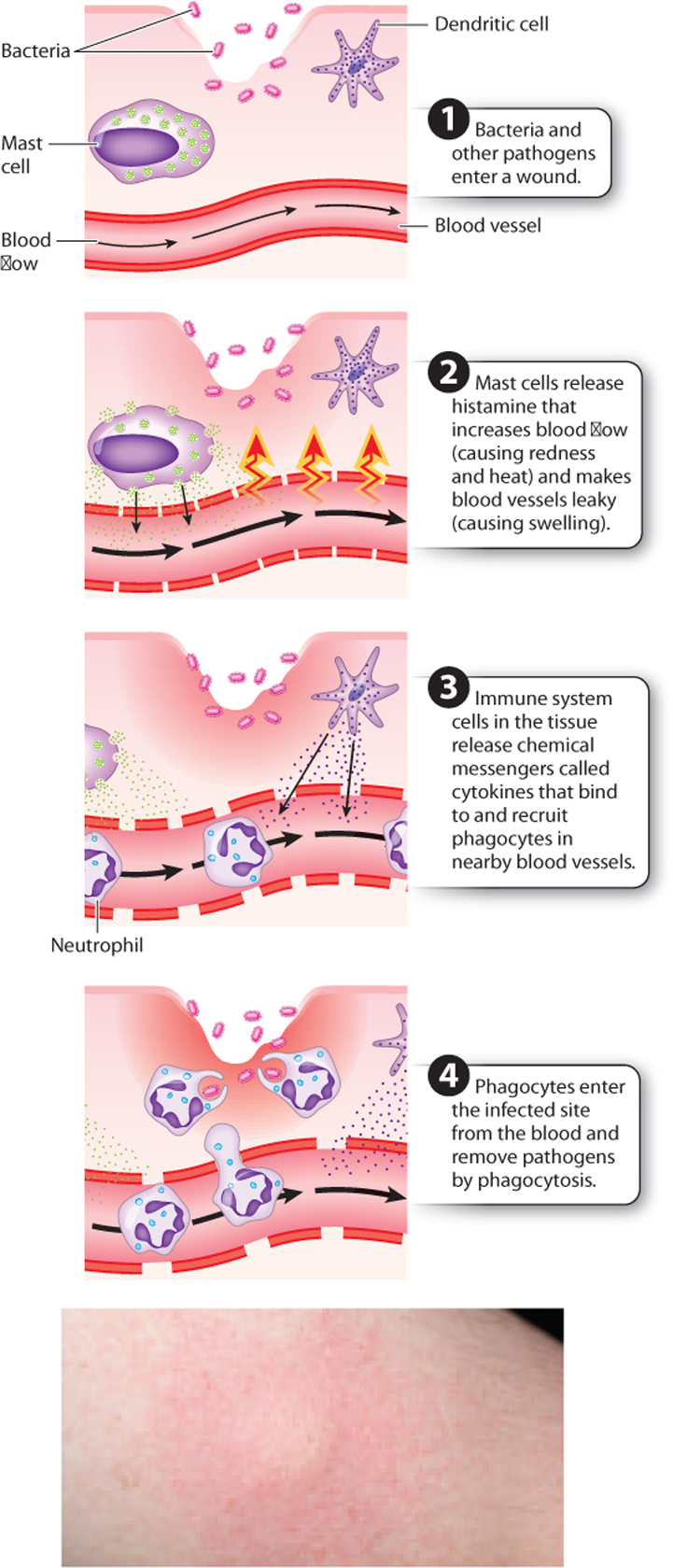
While there are generally some immune cells already present at the site of tissue injury or infection, many more must be recruited to fight infection successfully. That recruitment happens as part of a process called inflammation. The redness and swelling you see following an injury, a break in the skin, or an infection are signs of inflammation. More precisely, inflammation is a physiological response of the body to injury that removes the inciting agent if present and begins the healing process. It works through the coordinated responses of the local tissue, the vascular system (blood and lymph), and the innate immune system. Inflammation therefore is a useful, beneficial process. However, left unchecked or allowed to continue for a long time, it can be debilitating.
Inflammation is characterized by four classic signs, described by their Latin names: rubor (redness), calor (heat), dolor (pain), and tumor (swelling). The process begins following tissue injury or infection (Fig. 43.5). Certain cells in the tissue, such as dendritic and mast cells, are activated and release cytokines and other chemical messengers. Some of these chemical messengers recruit additional white blood cells to the site of infection or injury. Others make it easier for white blood cells to reach this site quickly. For example, histamine, released by mast cells and basophils, acts directly on blood vessels to cause vasodilation, increasing blood flow to the site of infection or injury. Vasodilation causes the characteristic redness and heat of inflammation. In addition, histamine increases the permeability of the blood vessel wall. Fluid leaks out of the blood vessel, carrying white blood cells into the damaged tissue. The increased fluid in the tissue surrounding the blood vessels is visible as swelling. Some chemical messengers act directly on nerve fibers, causing pain.
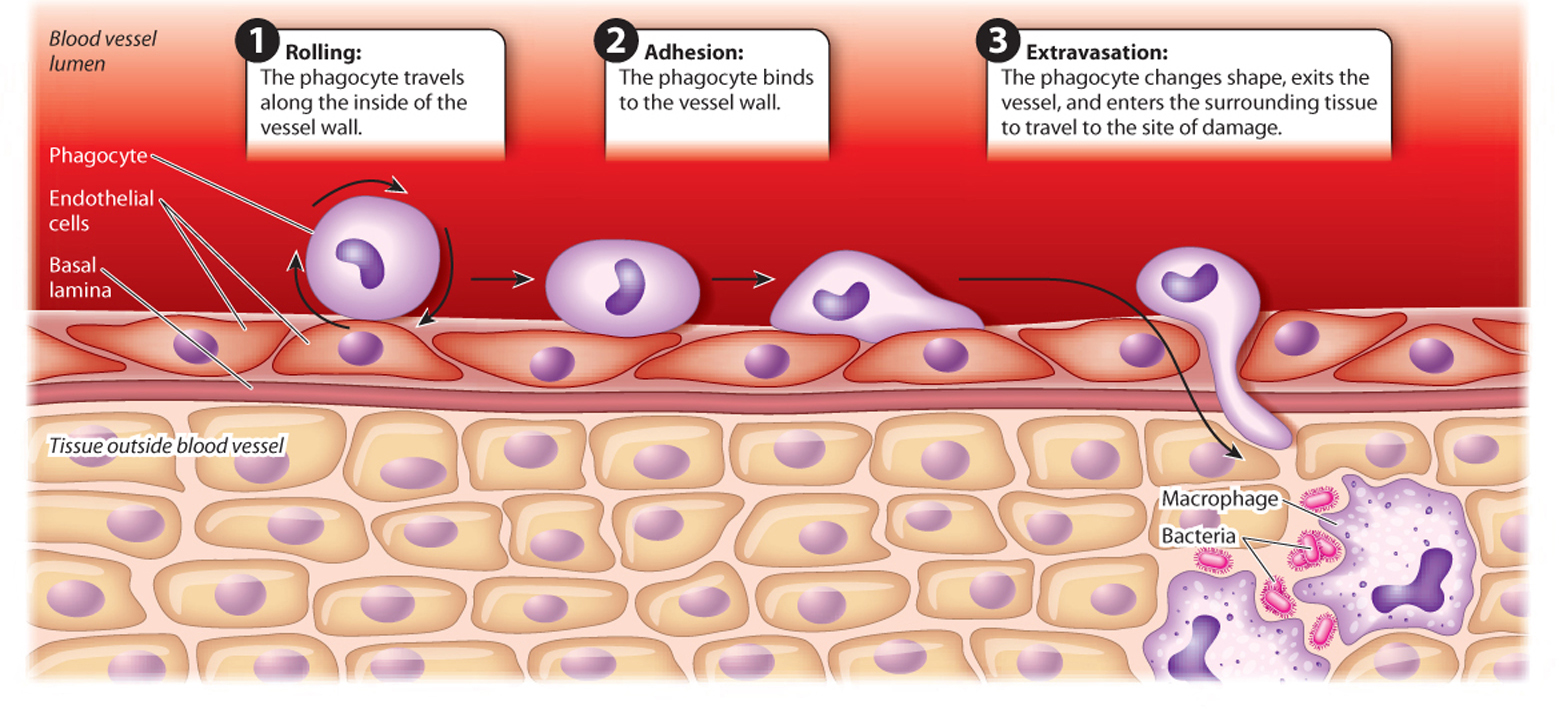
Phagocytes that travel in the blood can move from a blood vessel to the site of infection. This process is called extravasation (Fig. 43.6). The phagocyte travels along the vessel wall in a rolling motion, grabbing weakly onto the wall as glycoproteins on the phagocyte surface bind transiently to proteins on the endothelial cells. Stronger interactions put a brake on this rolling motion and allow the phagocyte to adhere more firmly to the vessel wall. As it nears the site of infection, the phagocyte encounters and binds to cytokines. The phagocyte then changes shape and moves in between cells lining the blood vessel and into the surrounding tissue.
Several proteins play key roles in inflammation. In the early 1900s, researchers studied patients with pneumonia caused by the bacterium Streptococcus pneumoniae. They looked for and found a change in the level of proteins in the blood as a marker for the initial phase of the disease, which became known as the acute phase response. One of these proteins, C-reactive protein or CRP, recognizes and binds to molecules on the surface of some bacteria. These CRP-coated bacteria are more easily taken up by phagocytes. The binding of a molecule like CRP to a pathogen to facilitate uptake by a phagocyte is called opsonization.
43.1.5 The complement system participates in the innate and adaptive immune systems.
In addition to individual proteins, such as CRP, there is an entire system of proteins circulating in the blood that participates in innate immune function. Collectively, these proteins make up the complement system. First described by Paul Ehrlich in the late 1800s, this system is so named because it supplements or complements other parts of the immune system.
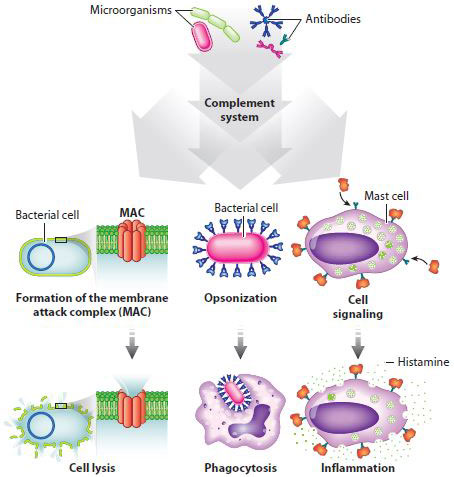
The complement system consists of more than 25 proteins that circulate in the blood in an inactive form (Fig. 43.7). The system is activated when these proteins bind to molecules specific to microorganisms or to antibodies (discussed below). Activation in turn sets off a biochemical cascade in which the product of one reaction is the enzyme that catalyzes the next. Such sequential activation leads to amplification of the response, as we saw earlier in cell signaling (Chapter 9) and in the endocrine system (Chapter 38), where a small stimulus can have a dramatic response.
Activation of the complement system has three effects. The most dramatic is breaking open cells, or lysis. Lysis results when complement proteins form a membrane attack complex (MAC) that makes holes in bacterial cells. Second, the complement system targets pathogens for phagocytosis by coating bacteria with a protein that phagocytes recognize (opsonization). Finally, activation of the complement system leads to the production of activated proteins that attract other components of the immune system. For example, some of these proteins stimulate mast cells, which release histamine and initiate the inflammatory response.
You can appreciate the importance of the complement system in the immune system by considering what occurs in individuals who lack key components. Complement deficiencies are a rare form of immunodeficiency, any disease in which part of the immune system does not function properly. Their effects depend on which component of the complement system is lacking. For example, one type of complement deficiency makes individuals vulnerable to bacterial infections because fewer pathogens undergo cell lysis and opsonization.
Because antibodies are an important signal activating the complement system, the complement system provides an important link between the innate, nonspecific arm of the immune system and the adaptive, specific arm, a topic we consider next.