43.4 THREE INFECTIONS: A VIRUS, BACTERIUM, AND EUKARYOTE
Although we have presented the different components of the immune system one at a time, in life they work together to provide a coordinated response to pathogens. We have seen how B cells often require T cell help, how the complement system can be activated by antibodies produced by B cells, and how macrophages present antigens in association with MHC molecules to T cells. In turn, pathogens have evolved means of evading the immune system. In this section, we consider the flu virus, the bacterium that causes tuberculosis, and the malaria parasite. These three pathogens illustrate how pathogens evolve and adapt in response to the immune system, and how the immune system in turn evolves and adapts in response to pathogens.
43.4.1 The flu virus evades the immune system through antigenic drift and shift.
Everyone knows the symptoms: fever, chills, sore throat, cough, weakness, and fatigue, perhaps a headache and muscle pain. Many viruses cause these common flu-like symptoms. However, the common cold, stomach flu, and 24-hour flu are caused by unrelated viruses, not by the flu virus.
The flu—its full name is influenza—is caused by an RNA virus that infects mammals and birds. It is notable for causing seasonal outbreaks, or epidemics. Four times in the last century—the Spanish flu of 1918, the Asian flu of 1957–58, the Hong Kong flu of 1968–69, and the swine flu of 2009—the virus spread more widely and infected more people, and the disease reached pandemic levels. The flu is more than just a nuisance: It causes hundreds of thousands of deaths each year, and millions of deaths during pandemics.
Part of the success of the virus lies in its ability to spread easily. The flu virus can spread in the air by small droplets produced when an infected person coughs or sneezes. It can also spread by direct contact or by touching a contaminated surface, such as a doorknob. Hand washing, therefore, is one of the most effective means of protection.
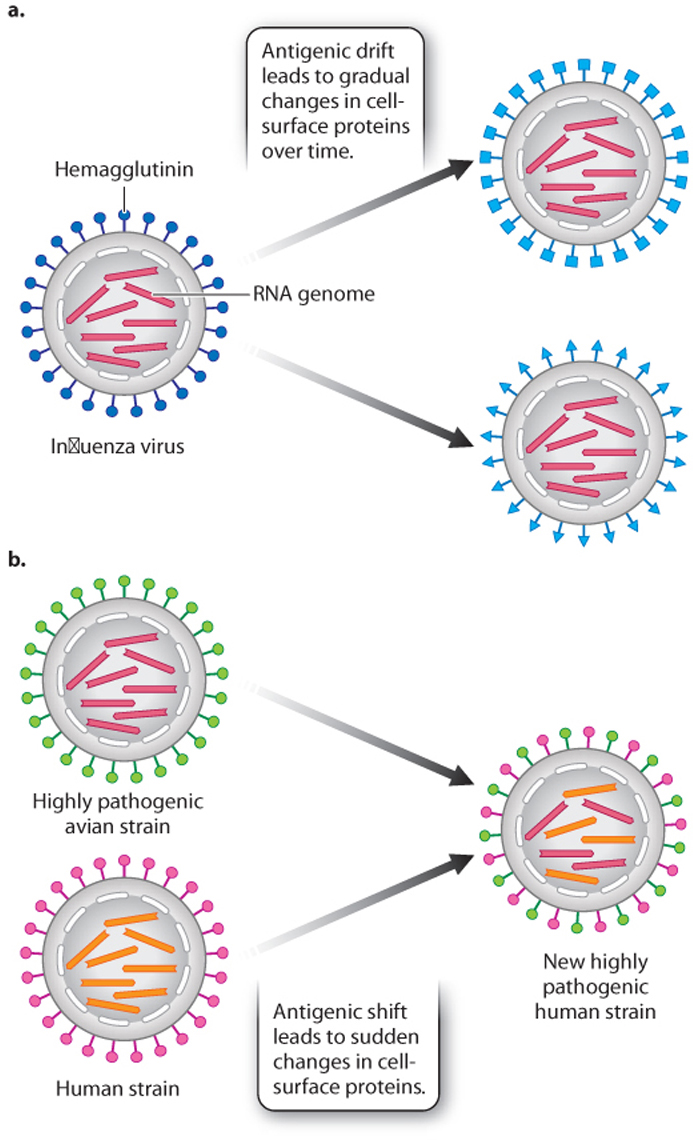
Another characteristic of the flu is its ability to evade the immune system. Like the Greek soldiers in their wooden horse, the flu virus can change its outer coat so memory cells, produced from earlier infections, no longer recognize it. There are three major types of flu virus—called A, B, and C—and many different strains. For example, the swine flu pandemic of 2009 was caused by a type A flu virus of the strain H1N1. These strains differ in several respects, including the structure of a cell-surface glycoprotein called hemagglutinin (HA). HA binds to epithelial cells and controls viral entry into these cells. It can bind only to cells that display a complementary cell-surface protein. As a result, hemagglutinin determines in part which organisms the flu virus infects—humans, pigs, or birds—and which part of the body it infects—the nose, throat, or lungs.
Cells infected by viruses secrete cytokines that bring macrophages, T cells, and B cells to the site of infection. These cytokines, produced in abundance, lead to many of the symptoms commonly associated with the flu. For example, the cytokine interleukin-1 produces fever.
Once in the cell, the virus replicates its genome and makes more virus particles. However, replication is prone to error, so there is a high rate of mutation that leads to changes in the amino acid sequences of antigens present on the viral surface, including HA. This process is called antigenic drift (Fig. 43.18a). It allows a population of viruses to evolve over time and evade memory T and B cells that remember past infections.
Antigenic drift leads to a gradual change of the virus over time. The flu virus is also capable of sudden changes by a process known as antigenic shift (Fig. 43.18b). The viral genome consists of eight linear RNA strands. If a single cell is co-infected with two or more different flu strains, the RNA strands can reassort to generate a new strain. H1N1, for example, has genetic elements from human, pig, and bird flu viruses. Antigenic drift and shift make it difficult to predict from year to year which strains will be most prevalent and therefore what vaccine will be most effective.
43.4.2 Tuberculosis is caused by a slow-growing, intracellular bacterium.
Our second example is tuberculosis, also known as TB. Like the flu, it is spread through small droplets dispersed in the air when a person with active disease coughs or sneezes. Also like the flu, the TB pathogen replicates inside host cells. But unlike the flu, TB is caused by a bacterium, not a virus. TB results from infection with Mycobacterium tuberculosis, a bacterium with several unusual properties. It is very small with a compact genome, lacks a plasma membrane outside of the cell wall, and replicates exceedingly slowly. M. tuberculosis takes about 16–20 hours to divide, compared to 20 minutes for the common intestinal bacterium and model organism E. coli.
TB is very common. It is estimated that about a third of the world’s population has TB. Symptoms are absent in most cases because the bacteria are in a dormant state, kept in check by the immune system. About 10% of cases are active, characterized by chronic cough, fever, night sweats, and the weight loss that gives TB its other name: consumption.
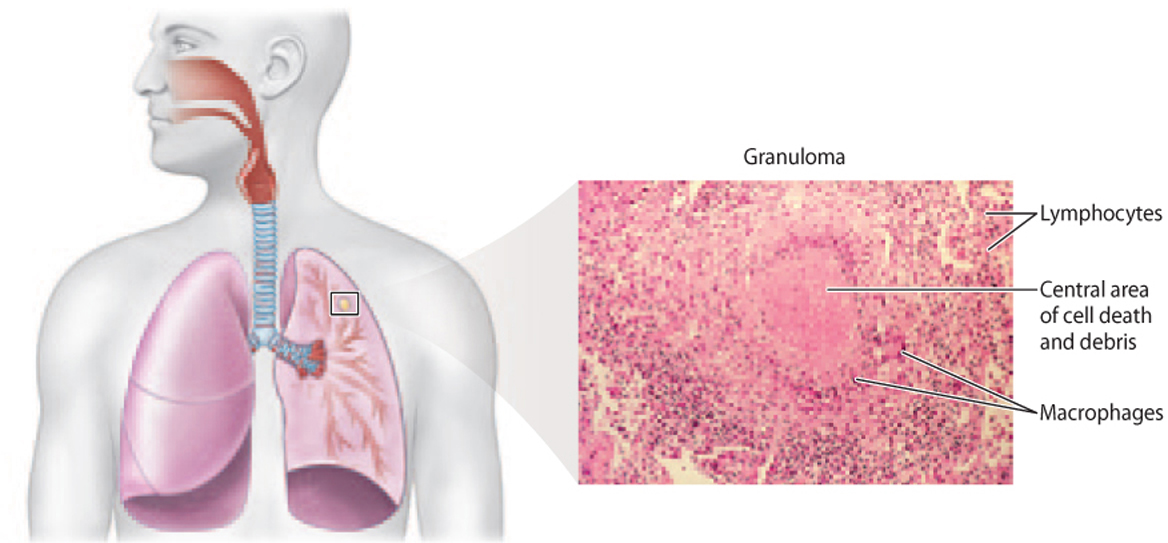
TB primarily affects the lungs. The bacteria enter macrophages in the alveoli of the lungs, where they replicate. The macrophages release cytokines, recruiting T and B cells to the site of infection. These lymphocytes surround the infected macrophages, forming a structure called a granuloma (Fig. 43.19). The granuloma helps to prevent the spread of the infection and aids in killing infected cells.
Many TB cases remain asymptomatic. However, the bacteria can sometimes overcome host defenses, especially if the immune system is compromised or suppressed, as in the case of co-infection with HIV. Treatment for active disease requires a long course of multiple antibiotics. In many areas of the world, however, TB has evolved antibiotic resistance, a growing and alarming problem.
Diagnosis poses challenges, especially in the case of asymptomatic infections. The tuberculin skin test is performed by injecting protein from the bacteria under the skin and observing whether or not a reaction, in the form of a hard, raised area, occurs. A positive skin test is an example of a delayed hypersensitivity reaction caused by the recruitment of memory T cells to the site of inoculation. Unfortunately, the test is not always accurate, and newer tests are being developed.
Developing an effective vaccine is challenging. The only one currently available is BCG (Bacillus Calmette-Guérin, named for Albert Calmette, a French bacteriologist, and Camille Guérin, a French veterinarian, who developed it early in the twentieth century). BCG is a live but weakened strain of a related bacterium that infects cows. In fact, this vaccine was developed following the success of using cowpox to immunize against smallpox. However, BCG is only sometimes effective, and a public health disaster resulting from contaminated vaccine occurred in 1930 in Germany. Consequently, it is not used for mass immunization in the United States.
43.4.3 The malaria parasite uses antigenic variation to change surface molecules.
“Bad air.” That’s the literal translation of “malaria,” a contraction of the Italian mala (“bad”) and aria (“air”). But malaria is not caused by the air. Nor is it caused by a virus or bacterium. Malaria is caused by a single-celled eukaryote of the genus Plasmodium (Case 4: Malaria). Several species infect humans, but P. falciparum is the most common and most virulent.
Malaria is both devastating and common: There are about 500 million cases and 2 million deaths every year, mostly in sub-Saharan Africa, and mostly of children. Malaria causes cyclical fevers and chills and can lead to coma and even death.
The malaria parasite is transmitted to humans by the bite of an infected mosquito (Fig. 43.20a). Using mosquitoes as a vector, the malaria parasite deftly bypasses the natural protective barrier provided by the skin. The malaria parasite spreads to the liver and then to red blood cells, where it completes its life cycle. The progeny can be taken up again by mosquitoes.
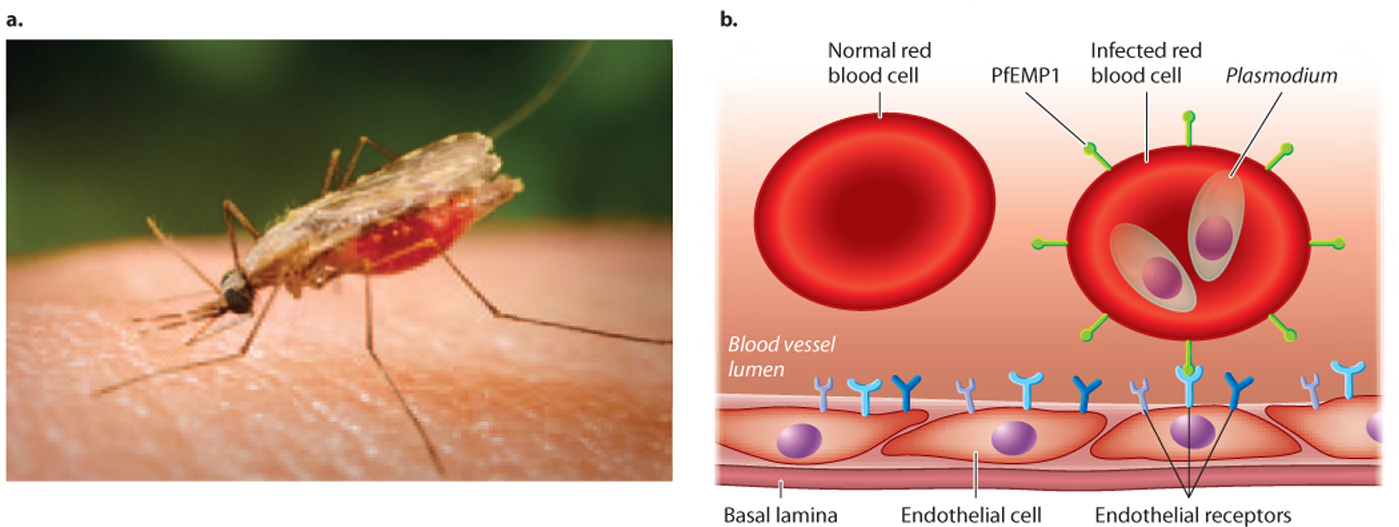
Malaria is an ancient parasite that has coevolved with humans (Case 4: Malaria). We have already seen how altered hemoglobin provides some protection against infection (Chapter 21). But the malaria parasite, too, has adapted—it is able to evade our immune system. It resides in liver cells and red blood cells, where it cannot be easily detected by macrophages, B cells, or T cells. Infected red blood cells can be removed by the spleen, but the malaria parasite has evolved a way to avoid this filtering action. It expresses an adhesive protein that inserts itself on the surface of red blood cells. This protein interacts with proteins on the surface of cells lining blood vessels, helping infected cells stick to blood vessel walls and keeping them in the circulatory system and out of the spleen (Fig. 43.20b). The protein, PfEMP1 (Plasmodium falciparum erythrocyte membrane protein 1), could conceivably be a target for antibodies or TCRs. However, it is encoded by one of about 60 genes, each slightly different from the others. The parasite expresses just one of these genes at a time and can change which one is expressed, in a process called antigenic variation. The great and ever-changing diversity of this protein, both in a single parasite and in the population as a whole, makes it a moving target for the immune system.
Despite continuing research, there is no vaccine. Researchers hope that the sequence of the malaria genome, completed in 2002, will provide new targets for vaccine research. In the meantime, efforts to control malaria focus on measures to control mosquitoes, such as nets and insecticides, and on treatment with antibiotics. The evolution of antibiotic resistance, however, is an all too familiar problem.