44.1 A TREE OF LIFE FOR MORE THAN A MILLION ANIMAL SPECIES
As discussed in Chapter 23, it is relatively easy to understand how the visible features of humans, chimpanzees, and gorillas show them to be more closely related to one another than any of them is to other animal species. Furthermore, it isn’t hard to see that humans, chimpanzees, and gorillas are more closely related to monkeys than they are to lemurs, and that humans, chimpanzees, gorillas, and monkeys are more closely related to lemurs than to horses. Anatomy and morphology reveal key evolutionary relationships among vertebrate animals, but how do we come to understand the place of vertebrates in a broader tree that includes all animals? More generally, how can we construct an animal phylogeny that includes organisms with body plans as different as those of sponges, sea stars, earthworms, and mussels?
44.1.1 Phylogenetic trees propose an evolutionary history of animals.
The anatomy of animals ranges from simple (think of sponges) to remarkably complex (lobsters, for example, or humans). All animals are multicellular heterotrophs, gaining both food and energy from organic molecules (Chapter 6). Most fungi are also multicellular heterotrophs (Chapter 34), but fungi have cell walls and animals do not. Animals are also distinguished by embryos that include a gastrula stage (Chapter 42) and by the presence of collagen, a fiberlike protein that makes up connective tissues such as basal lamina, tendon, and cartilage.
While they share some general features, animals vary widely in many respects. For example, they differ in the ways that they gather food. Tiny animals called placozoans that live on the seafloor absorb organic compounds directly from the environment and take in particulate matter by phagocytosis. In contrast, bilaterally symmetrical animals from insects to eagles have highly adapted nervous and muscular systems that enable them to capture plants or other animals and digest them in a specialized gut.
Sponges have no nerve cells, and sea anemones have only simple networks of nerves, but humans have brains containing trillions of cells that permit us to make and communicate discoveries about sponges, sea anemones, and other organisms we encounter. How the diverse morphologies, anatomies, and behaviors that characterize animal species came to be is a question of evolution, and phylogenetic trees provide hypotheses about the evolution of animal diversity.
Early in the history of biology, taxonomists such as Linnaeus recognized that for all their diversity, animals can be sorted into a limited number of distinctive body plans, each group today formally called a phylum (plural, phyla). The problem for biologists has long been how to understand the evolutionary relationships among animal phyla, still an active field of research today.
Fig. 44.1 shows a simple phylogenetic tree. Recall the basic logic of phylogenies, presented in Chapter 23. The fundamental mechanism by which biological diversity increases is speciation, the divergence of two populations from a common ancestor. Played out repeatedly through time, speciation gives rise to a treelike pattern of evolutionary relatedness, with more closely related taxa branching from points close to the tips of the tree and more distantly related taxa diverging from branch points nearer its base. Distinctive features of organisms, called characters, evolve throughout evolutionary history, and the time when they arose can be estimated from their shared presence in the descendants of the population in which the character first evolved. But a prominent feature of evolution is change, and in fact many characters change from one form to another and sometimes back again. Thus, mammals are descended from egg-laying ancestors, but most living mammals do not lay eggs—that character has been lost, replaced by live birth.
A character can evolve more than once in separate groups by convergent evolution. Such characters, called analogies, include the single-lens eye in octopus and vertebrates, and wings in birds and bats. These characters are not helpful in reconstructing evolutionary relationships. Close studies of morphological and molecular characters can often distinguish analogies from similarities that result from common ancestry, called homologies, which are useful in understanding evolutionary relationships. A large number of studies have resolved many but not all of the evolutionary relationships among animals.
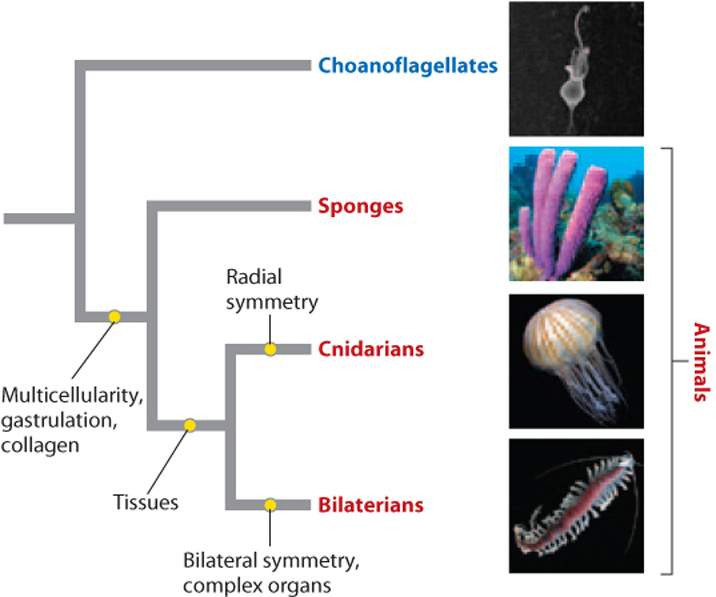
With these points in mind, Fig. 44.1 proposes that animals are closely related to protists called choanoflagellates (Chapter 27), but differ from them in the presence (among other features) of persistent multicellularity, the formation of a gastrula during early development, and the synthesis of collagen. Poriferans (the phylum that is made up of sponges) diverged from other animals early, and cnidarians (the phylum that contains jellyfish and sea anemones) diverged later from animals whose descendants have well-defined and complex organs. Note that this diagram does not tell us that sponges are older than more complex animals. Instead, it says that sponges and more complex animals diverged from a common ancestor but doesn’t tell us what that common ancestor looked like. Similarly, cnidarians and more complex animals with bilateral symmetry diverged from their last common ancestor at a single point in time and so are equally old, but structurally distinct.
We’ll use phylogeny as our framework for discussing the immense diversity of animals, but first, we need to consider how the tree itself has taken shape through more than a century of biological research.
44.1.2 Ninetheenth-century biologists grouped animals by anatomical and embroyological features.
Since the earliest days of comparative anatomy, it has been clear that animals vary in their structural complexity. Sponges have only a few cell types, and those cells are not organized into tightly coordinated tissues or organs. Jellyfish and sea anemones have simple tissues but not complex organs. Crabs and alligators have complex organs that govern movement, behavior, digestion, and gas exchange.
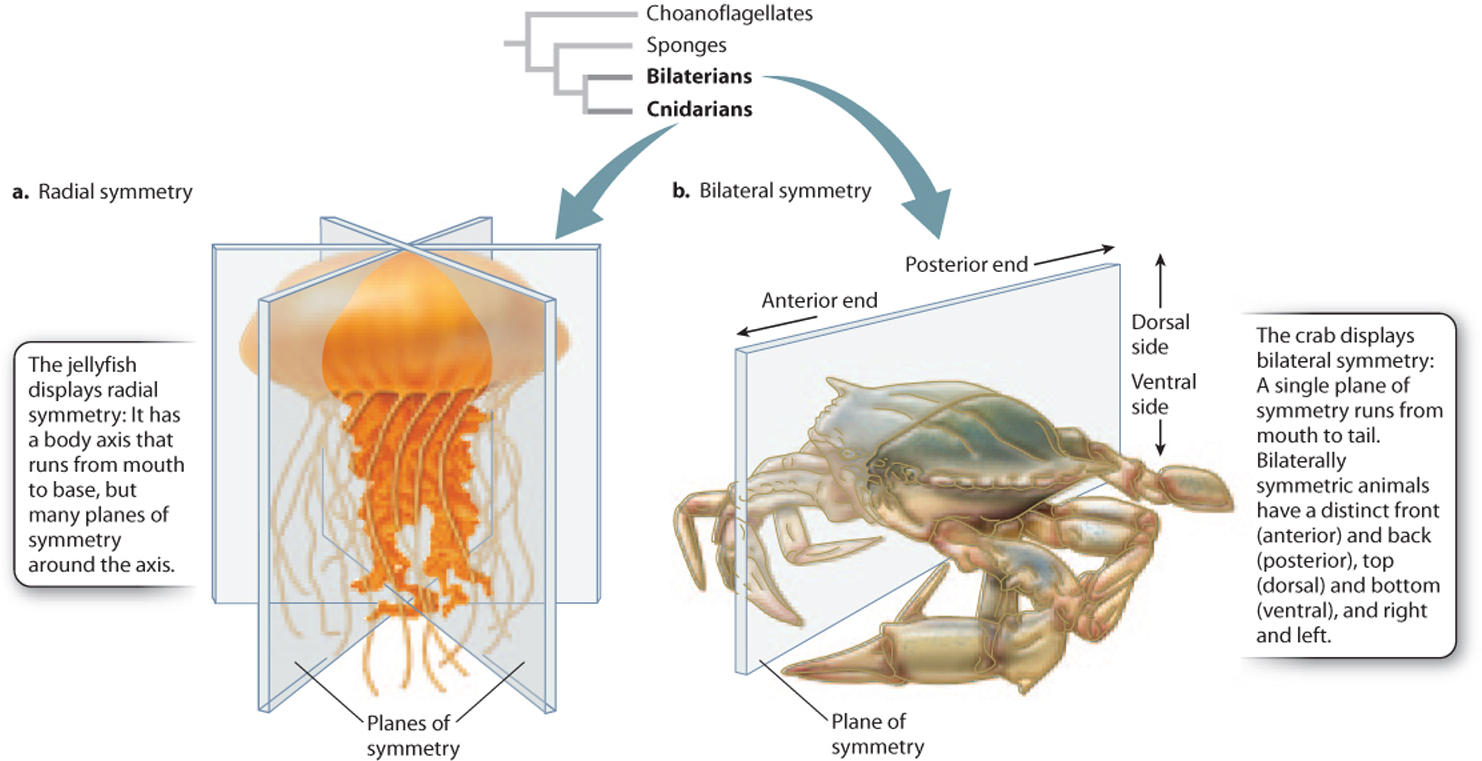
Organisms also differ in their symmetry. Sponges are often very irregular in form. Jellyfish and sea anemones display radial symmetry, meaning that their bodies have an axis that runs from mouth to base with many planes of symmetry through this axis (Fig. 44.2a). This organization allows jellyfish to move up and down in the water column by flexing muscles around their bell-like bodies, and permits sea anemones and corals to wave their ring of food-gathering tentacles in all directions at once. Most simple animals with radial symmetry are grouped together on one branch of the animal tree, in the phylum Cnidaria.
Other organisms show bilateral symmetry: Their bodies have a distinct head and tail, marking front and back, with a single plane of symmetry running between them at the midline (Fig. 44.2b). Bilateral symmetry enables animals to move in one horizontal direction to capture prey, find shelter, or escape from enemies. It also allows the development of specialized sensory organs at the front end for guidance, and specialized appendages along both sides for locomotion, sensing the environment, or defense. Animals with bilateral symmetry cluster together on one branch of the animal tree and are called Bilateria.
Biologists understood early that cnidarians are simpler than animals with bilateral symmetry. Bilaterian animals develop, to varying degrees, digestive and circulatory systems, nervous systems, and muscles. From these observations, early biologists concluded that sponges branched near the base of the animal tree, cnidarians in the middle, and bilaterians closer to the top.
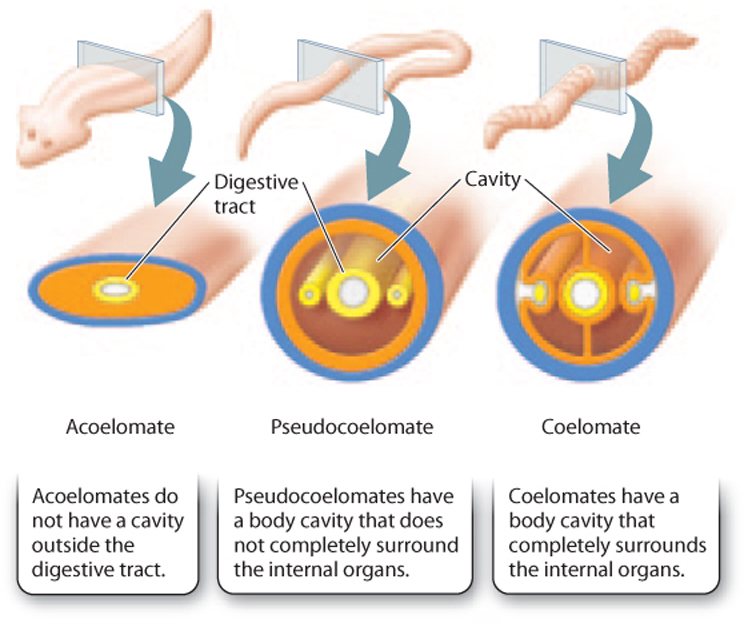
Within the bilaterian group, however, understanding evolutionary relationships proved more difficult. Some biologists pointed to the presence or absence of a cavity, or coelom, in tissues surrounding the gut, dividing bilaterians into three groups: acoelomates (without a body cavity), coelomates (with a body cavity), and pseudocoelomates (with a body cavity that does not completely surround the internal organs). These groups are shown in Fig. 44.3. A fluid-filled body cavity cushions the internal organs against hard blows to the body and enables the body to turn without twisting these organs. A body cavity also allows internal organs like the stomach to expand, enhancing digestive function.
Early biologists also noted that some animals, notably insects and earthworms, show a striking pattern of body segmentation, but others do not. These variations in anatomy were critically important in defining phyla within bilaterian animals, but in the end they did not provide a way to understand the details of evolutionary relationships among these phyla.
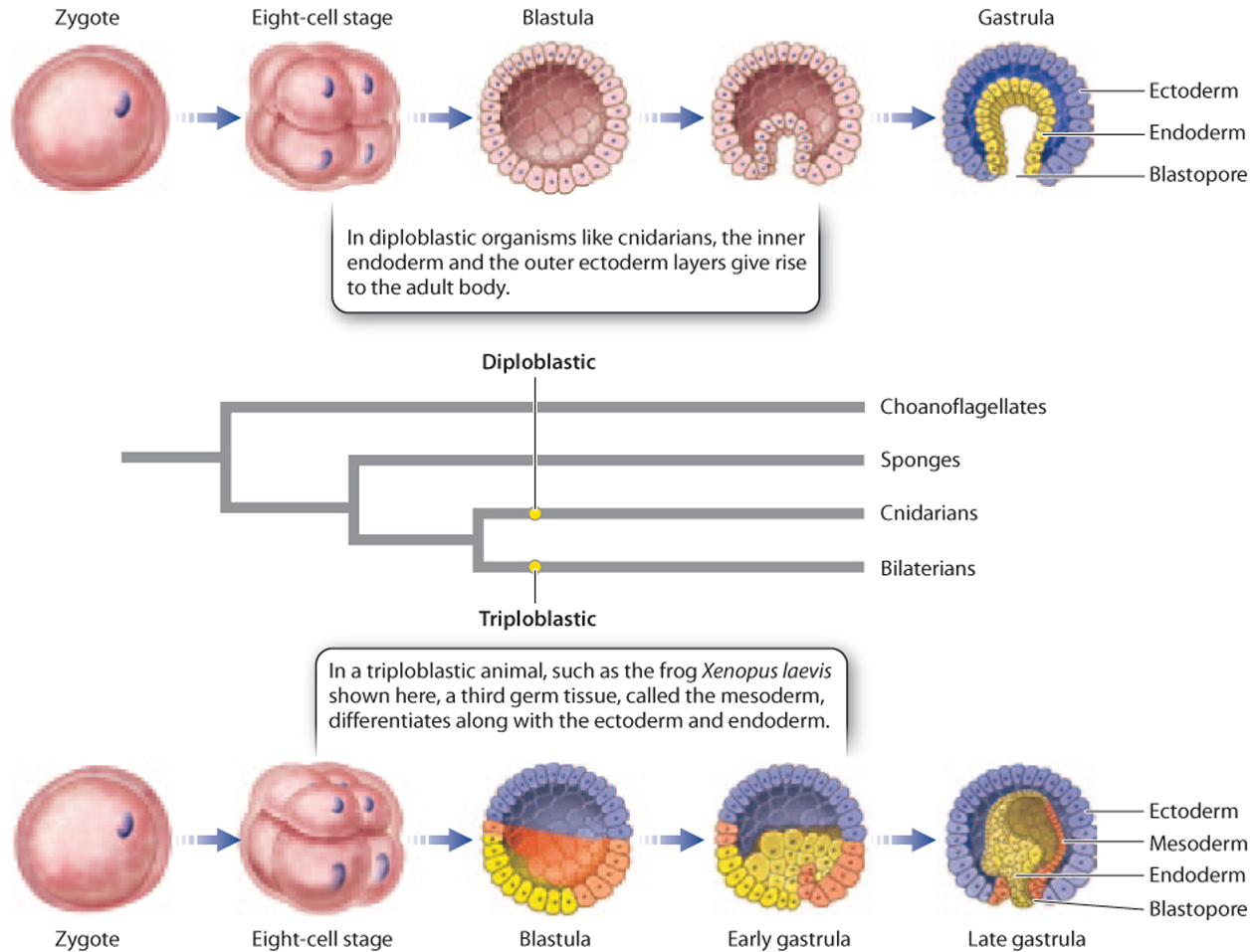
New insights became possible with the advent of microscopes that enabled the direct study of development from its earliest stages. One important observation was that some animals that look very different as adults share patterns of early embryological development. For example, adult sea stars and catfish have few morphological features in common, but their embryos show a number of key similarities, including the number of embryonic tissue layers they develop and the way that the early cell divisions occur. In the Cnidaria, the embryo has two germ layers, termed endoderm and ectoderm, from which the adult tissues develop. These animals are therefore called diploblastic (Fig. 44.4). The bilaterian animals are triploblastic, with a third germ layer, the mesoderm, lying between the endoderm and ectoderm, and developing into muscles and connective tissues (Fig. 44.4).
Comparative embryology also enabled biologists to divide bilaterian animals into the two groups shown in Fig. 44.5, called protostomes (from the Greek for “first mouth”) and deuterostomes (from the Greek for “second mouth”). In protostomes, the first opening to the internal cavity of the developing embryo, called the blastopore, becomes the mouth. In deuterostomes, the blastopore becomes the anus. However, only in the age of molecular sequence comparisons have the relationships among phyla within each of these two groups become clear.
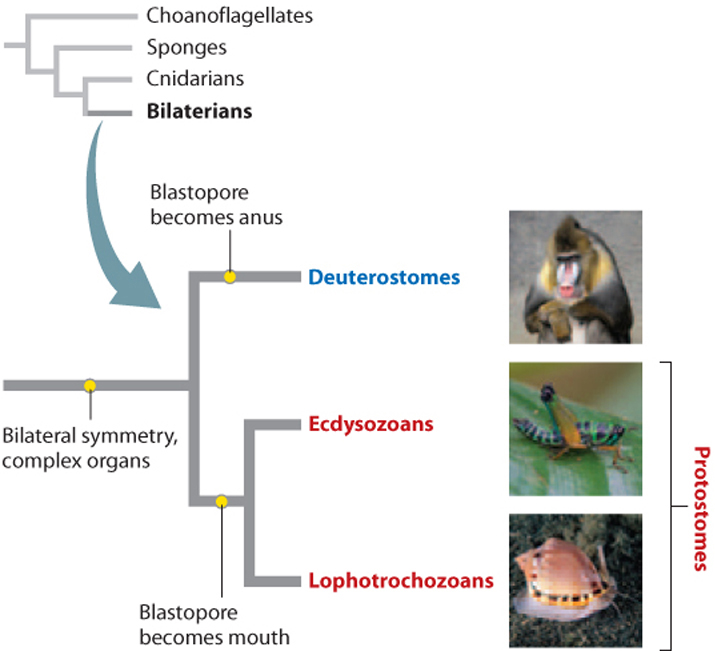
44.1.3 Molecular sequence comparisons have confirmed some relationships and raised new questions.
Over the past two decades, comparisons among DNA, RNA, and amino acid sequences have greatly improved our understanding of evolutionary relationships among major groups of animals. Molecular comparisons support many of the conclusions reached earlier on the basis of comparative anatomy and embryonic development, including the early divergence of sponges. Other hypotheses have been rejected. For example, the traditional phylogenetic division of bilaterians into acoelomate, coelomate, and pseudocoelomate groups gets little support from molecular studies. Neither do molecular sequence comparisons support the once widespread view that segmented bodies indicate a close relationship between earthworms and lobsters.
Some of the biggest surprises provided by molecular data concern the relationships of phyla within the protostomes, showing that the protostome phyla can be divided into two subgroups, the lophotrochozoans and the ecdysozoans (Fig. 44.5). As we will see, biologists have identified developmental and anatomical characters that unite the phyla on each branch, but the phylogenetic significance of these features was recognized only after gene sequences revised the thinking about protostome animals. Molecular sequence comparisons have also overturned traditional views on relationships among the deuterostome phyla, discussed later in this chapter.
Quick Check 1
Which animals are more closely related to sponges, Cnidaria or Bilateria?