44.2 THE SIMPLEST ANIMALS: SPONGES, CNIDARIANS, CTENOPHORES, AND PLACOZOANS
The human body is complex, our diverse functions made possible by sophisticated, interacting organ systems: from muscle pairs attached to skeletons that allow us to run, jump, and dance to lungs that exchange gases with the atmosphere and a brain and nervous system that enable us to sense our environment and respond to it. None of these organ systems is well developed in early-branching animal groups. How then do sponges, cnidarians, ctenophores, and placozoans function, and what are the consequences of their anatomical organization for diversity and ecology? On the other hand, these animals have survived a half billion years of Earth history. What can we learn from the diversity and continuity of their remarkable adaptations?
44.2.1 Sponges are simple and widespread in the oceans.
As we discussed in Chapters 27 and 28, the unicellular ancestors of animals carried out all functions from metabolism to reproduction as individual cells. The cells making up sponges retain much of this independence, while also having some benefits of multicellularity. Coordination among cells means that there can be distinct kinds of cell specialized for different functions—cells that form a protective skin, for example, and others that secrete enzymes for digestion. Most dramatically, multicellularity permits sponges to extend above the seafloor, giving them access to food suspended in water currents.
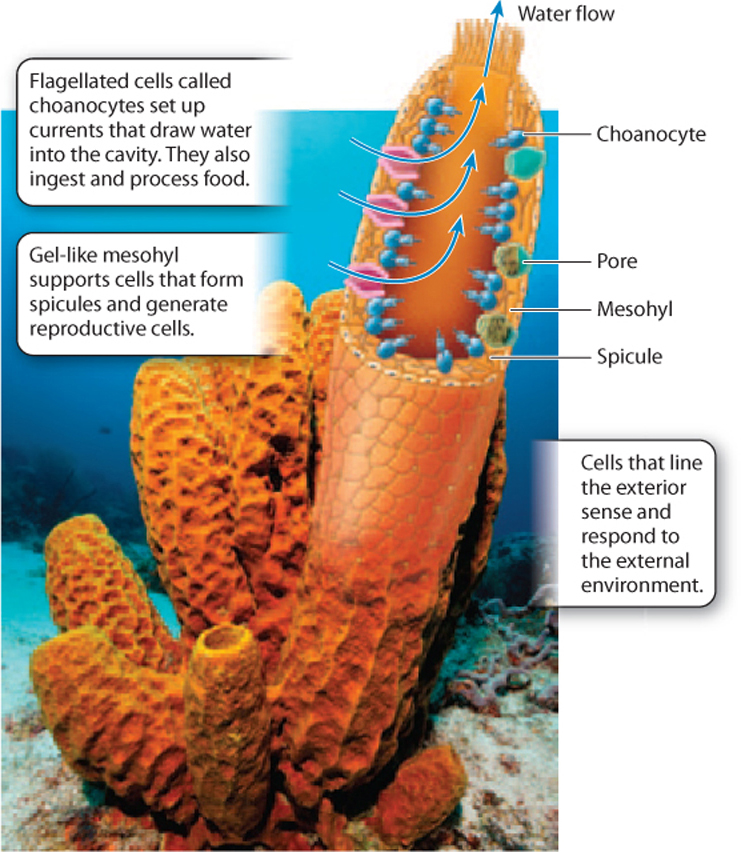
The sponge body plan resembles a flower vase, with many small pores along its sides and a larger opening at the top (Fig. 44.6). The cells on the outer surface of the sponge are tough and act as the sponge’s skin. The interior surface is lined by cells called choanocytes that have flagella and function in nutrition and gas exchange. Choanocyte cells have a collar of small cilia around their flagellum, much like the cells of our closest unicellular relatives, the choanoflagellates (Chapter 27). Between the interior and exterior cell layers lies a gelatinous mass called the mesohyl (Fig. 44.6). Mesohyl is mostly noncellular, but it contains some amoeba-like cells that function in skeleton formation and the dispersal of nutrients.
Sponges gain nutrition by intracellular digestion (Chapter 40). The choanocytes that surround the interior chamber of the sponge beat their flagella, creating a current that draws water from outside the body, through the pores in its walls and upward through the central cavity of the sponge, where it exits through the large opening at the top. The circulating water contains food particles and dissolved organic matter, which cells lining the cavity capture by endocytosis. The individual cells then metabolize the food in their interiors, much the way that protozoans feed.
From this observation, we might conclude that sponges are simply a group of uncoordinated cells, each working for itself, but that isn’t the case. The choanocyte flagella beat in a coordinated pattern, helping to draw water into the body interior through the pore system and outward again through the vase opening. Moreover, the shape of the sponge body itself directs water movement across feeding cell surfaces. These features effectively move water across the cells, facilitating food uptake. Sponge cells require oxygen for respiration and must get rid of the carbon dioxide respiration generates. Gas exchange occurs by diffusion, aided by the movement of water through the sponge cavity.
Sponges don’t have highly developed reproductive organs. Instead, cells recruited from the choanocyte layer migrate into the mesohyl, where they undergo meiosis and differentiate as sperm or eggs. Sperm released into the water fuse with eggs in the mesohyl of other sponges.
Many sponges build skeletons of simple structures called spicules. Some sponges precipitate spicules of glass-like silica (SiO2). Others, however, make their spicules—and sometimes massive skeletons—of calcium carbonate (CaCO3). Still other sponges have a skeleton made up of proteins (these are often used as bath sponges). So we see that sponges are intermediate in the sophistication of their bodies. Their cells function much as single-celled protozoa do, but they coordinate their activities and so are more efficient in extracting food and oxygen from seawater.
Despite their anatomic simplicity, sponges are major contributors to seafloor communities. Approximately 9000 species of sponge have been described, most of them ocean dwelling, but a few that live in freshwater lakes. Many sponges obtain at least part of their nutrition from symbiotic algae living within their bodies. In fact, all sponges are full of bacterial cells. Many of these are probably symbionts, but only a few experiments have demonstrated their function.
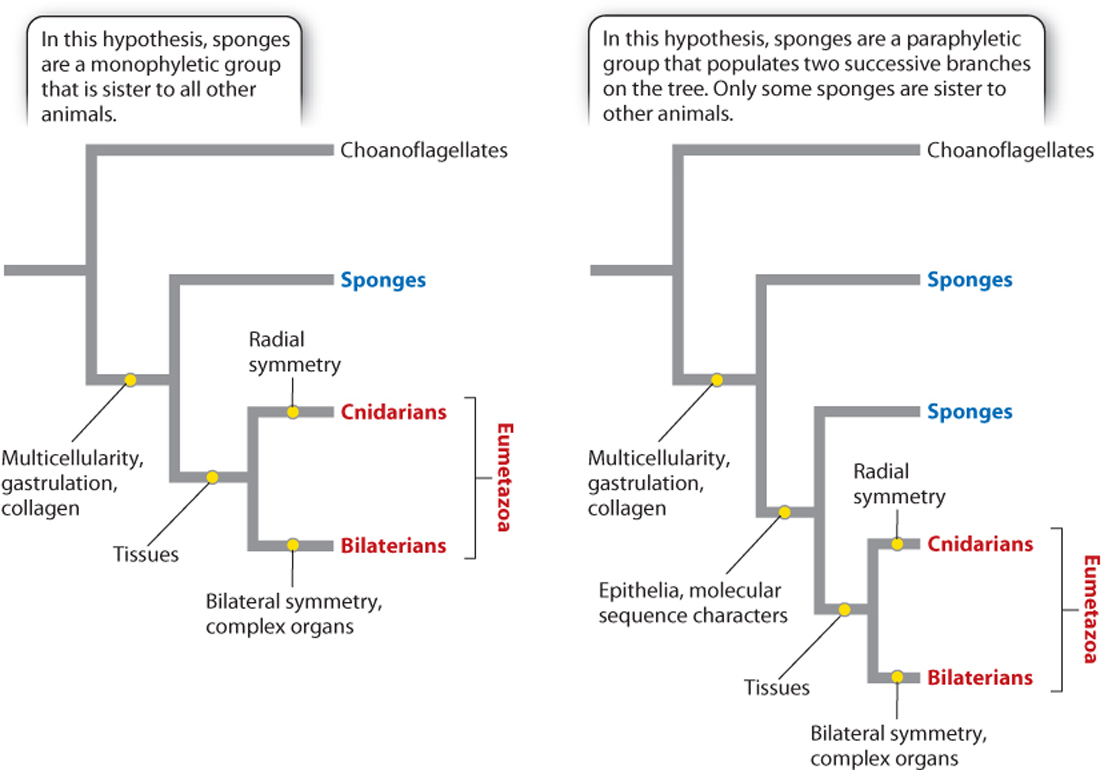
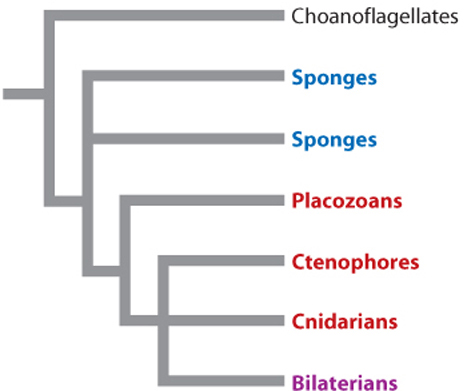
Until recently, most biologists regarded sponges as a monophyletic group, one that includes all the descendants of a common ancestor. Some molecular data, however, now suggest that sponges are paraphyletic—that is, that they contain some but not all the descendants of their common ancestor (Chapter 23).
Fig. 44.7 shows these two possibilities. If the tree on the left is correct, the two main limbs of the animal tree contain very different organisms—the simple sponges on one branch and all more complex animals on the other. As noted earlier, this figure indicates that sponges are distinct from other animals but leaves open the question of what ancestral animals were like. In contrast, the hypothesis illustrated by the tree on the right suggests that the last common ancestor of all living animals, in essence, was a sponge. At present, biologists are divided on this issue; more research is needed to demonstrate how sponges relate to each other and to other animals, which are grouped together as the Eumetazoa (Fig. 44.7).
44.2.2 Cnidarians are the architects of life's largest constructions: coral reefs.
Many of us have encountered at least a few cnidarians. Jellyfish flourish in marine environments from coastlines to the deep sea; sea anemones cling to the seafloor, extending their tentacles upward to gather food and deter predators; corals secrete massive skeletons of calcium carbonate, forming reefs that fringe continents and islands in tropical oceans.
Jellyfish and sea anemones look strikingly different, but they share a body plan common to all cnidarians (Fig. 44.8). At one end of the radially symmetrical body is a mouth surrounded by tentacles armed with stinging cells that subdue prey and defend against enemies. An anemone is like a jellyfish stuck upside down onto the seafloor with its tentacles and mouth facing upward. We call a free-floating jellyfish a medusa, and the sessile form of an anemone a polyp. Some cnidarians develop into one form or the other, but many have life cycles that alternate between the two.
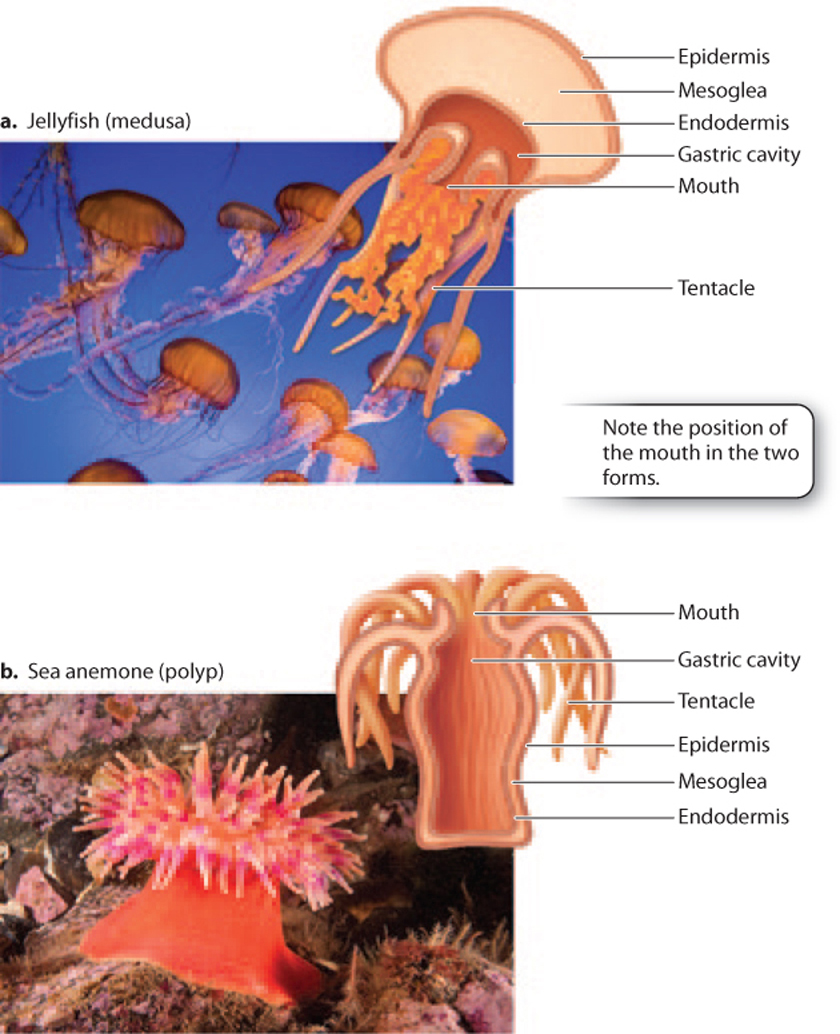
The mouth of both medusa and polyp opens into a closed internal gastric cavity, the site of extracellular digestion and excretion. Rather than digesting food particles inside individual cells, cnidarians receive both food and digestive enzymes in the gastric cavity. (The digestive enzymes are secreted by cells that line the walls of the cavity.) This arrangement permits cnidarians to digest large food items, such as a whole fish (paralyzed by the stinging cells on the tentacles), whose dissolved nutrients can then be absorbed through the walls of the cavity. Cnidarians, then, can consume many types of food that are unavailable to sponges.
The cnidarian body develops from a diploblastic embryo (one with two germ layers). It has an outer layer, the epidermis, which develops from the ectoderm, and an inner lining, the endodermis, that derives from the endoderm. These tissues enclose a gelatinous mass called the mesoglea (the “jelly” of jellyfish). This organization sounds a bit like the sponge body plan, but there are important differences.
First, in cnidarians, the cells that form the epidermis and endodermis occur as closely packed layers of cells embedded in a protein-rich matrix, forming an epithelium (Chapter 10). Epithelia line compartments (like the gut) within animal bodies, but they do not occur in most sponges. These tissues are formed from an unusually tight layer of specialized cells, each connected to its neighbors with specialized junctions that regulate the passage of ions or other molecules. Epithelial layers often absorb or secrete substances into the compartments they line, making possible organs such as those of the digestive system (Chapter 40).
Second, cnidarians have a wider array of cell types than sponges do, permitting more sophisticated tissue function. Muscle cells allow jellyfish to swim through the ocean. A simple network of nerve cells permits cnidarians to sense their environment and respond to it by directional movement. No such cells occur in sponges. Cnidarians don’t have a brain, but at least a few have light-sensitive cells that function as simple eyes.
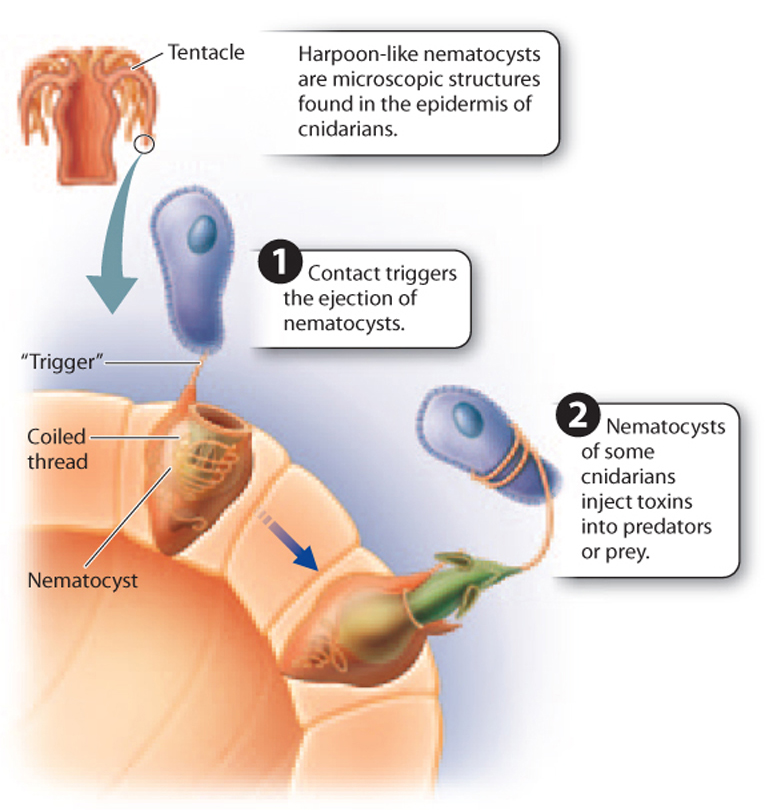
Moreover, whereas sponges filter water to gain food, cnidarians are predators, capturing prey with their tentacles and digesting it in the gastric cavity. Specialized cells on the tentacles contain a tiny harpoon-like organelle called a nematocyst, often tipped with a powerful neurotoxin that greatly aids prey capture and defense against other predators (Fig. 44.9). Other specialized cells lining the gastric cavity secrete digestive enzymes that break down ingested food into molecules that can be taken up by cells lining the cavity by endocytosis. There is no specialized passage for waste removal. Instead, waste is excreted back into the gastric cavity and leaves by way of the mouth. Oxygen uptake and carbon dioxide release occur by diffusion.
Many cnidarians reproduce asexually to form colonies. For example, corals form extensive rounded, fan-shaped, or hornlike colonies by budding, building potentially massive structures on the seafloor (Fig. 44.10a). In the Portuguese man-of-war, different individuals in the same colony develop distinct morphologies, some specialized for flotation, others for prey capture, and still others for reproduction (Fig. 44.10b). Among animals without complex organs, such colonies represent the height of morphological complexity.
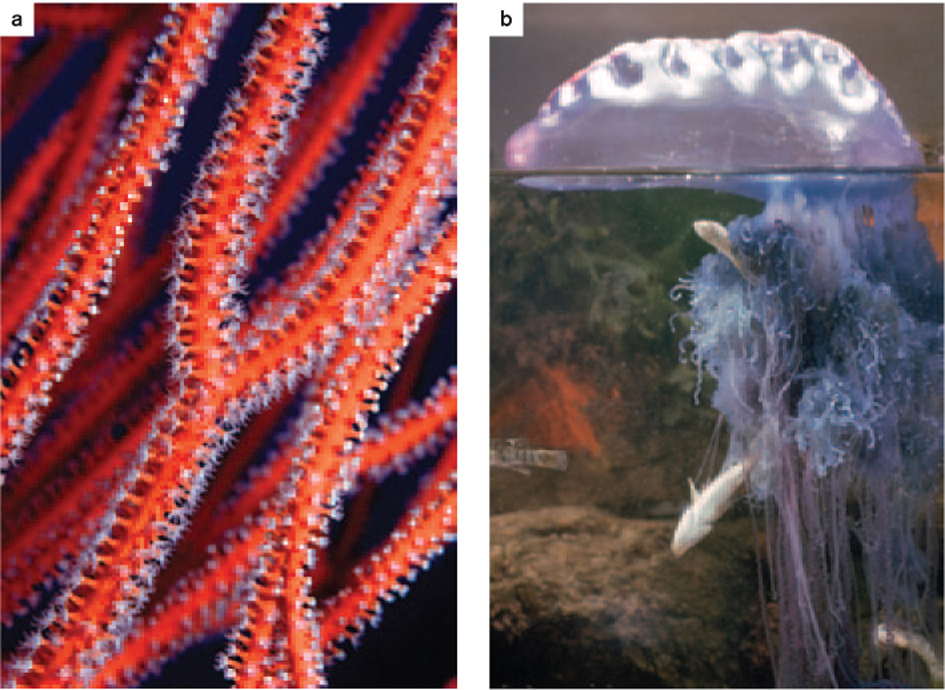
About 9000 species of cnidarians live in the oceans. Reef corals are remarkable not only for the size of their skeletal colonies, but also for their mode of nutrition. Most have lost the ability to capture prey and instead gain nutrition from symbiotic algae in their surface tissues.
44.2.3 Ctenophores and placozoans represent the extremes of body organization among early branching animals.
Ctenophores, or comb-jellies, resemble cnidarians in body plan, and for many years most biologists thought that the two were close relatives. Like cnidarians, comb-jellies have radial symmetry, with an outer epithelium and an inner endodermis that enclose a gelatinous interior (Fig. 44.11). Comb-jellies also differentiate muscle cells and a simple nerve net, as well as rudimentary gonads. They are predators that feed by ingestion, digesting prey within their gastric cavity by enzymes secreted from the cells lining the gut cavity. Gas exchange occurs by diffusion. However, there are important differences between cnidarians and comb-jellies.
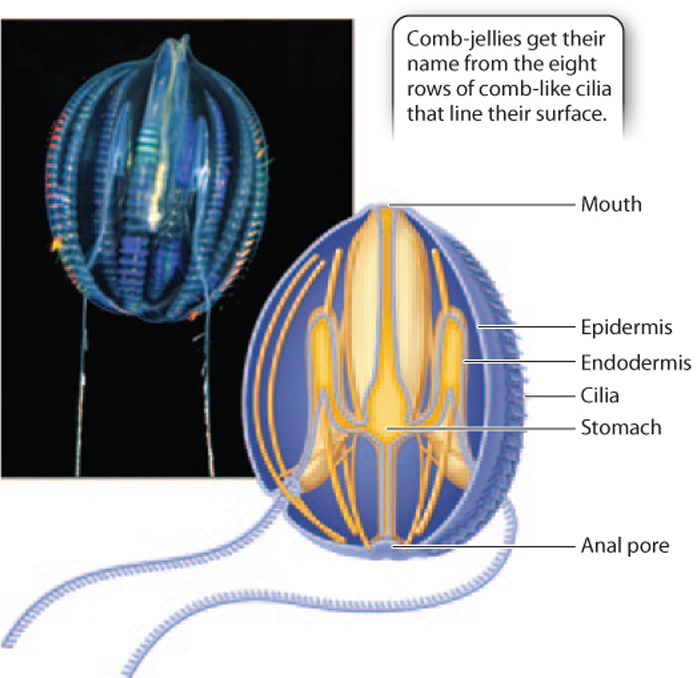
Comb-jellies propel themselves through the oceans by the coordinated beating of cilia that extend from epidermal cells. These cilia are usually arranged in comb-like groups, hence the common name for this phylum. Some but not all comb-jellies also have a pair of long tentacles that aid in feeding. Like cnidarians, comb-jellies have specialized cells on their tentacles, but in comb-jellies, the cells secrete adhesive molecules that entangle prey.
Unlike cnidarians, digestive wastes generated by comb-jellies move through a gut cavity for elimination through an anal pore opposite the mouth. This simple flow-through gut provides comb-jellies with an anterior–posterior axis that cnidarians do not have. This arrangement allows each section of the gut to be specialized for a particular function, such as temporary storage (near the mouth), digestion (in the middle), and absorption and excretion (at the rear), increasing the overall efficiency in processing food. Cnidarians digest and absorb and store food in the same pouch, and so are much less efficient.
Also, the pattern of muscle cell development in comb-jellies suggests that these cells originate from a rudimentary mesoderm situated between the ectoderm and endoderm. Flow-through guts, mesoderm, and axial symmetry are characteristic of bilaterian animals, as is another feature of comb-jellies—the use of acetylcholine as a neurotransmitter (Chapter 35). This unique combination of traits makes placement on the tree of life difficult. About 100–150 species of comb-jelly have been named, and photographs from deep-sea submersibles make it clear that many more remain to be discovered in the oceans.
Quick Check 2
Which would you expect to use available nutrients more effectively and reproduce more rapidly, a jellyfish or a sponge of the same size?
If comb-jellies are the most complex animals discussed so far, placozoans are the simplest. These tiny (millimeter-scale) animals each contain only a few thousand cells arranged into upper and lower epithelia that sandwich an interior fluid crisscrossed by a network of multinucleate fiber cells (Fig. 44.12). Placozoans can absorb dissolved organic molecules, but commonly feed by surrounding food particles and secreting digestive enzymes to break them down. Individual cells then bring food particles in by endocytosis. Placozoans have no specialized tissues and few differentiated cell types. Cilia on cell surfaces allow movement, and gas exchange occurs by diffusion. Placozoans reproduce asexually but can also form egg and sperm cells for sexual reproduction.
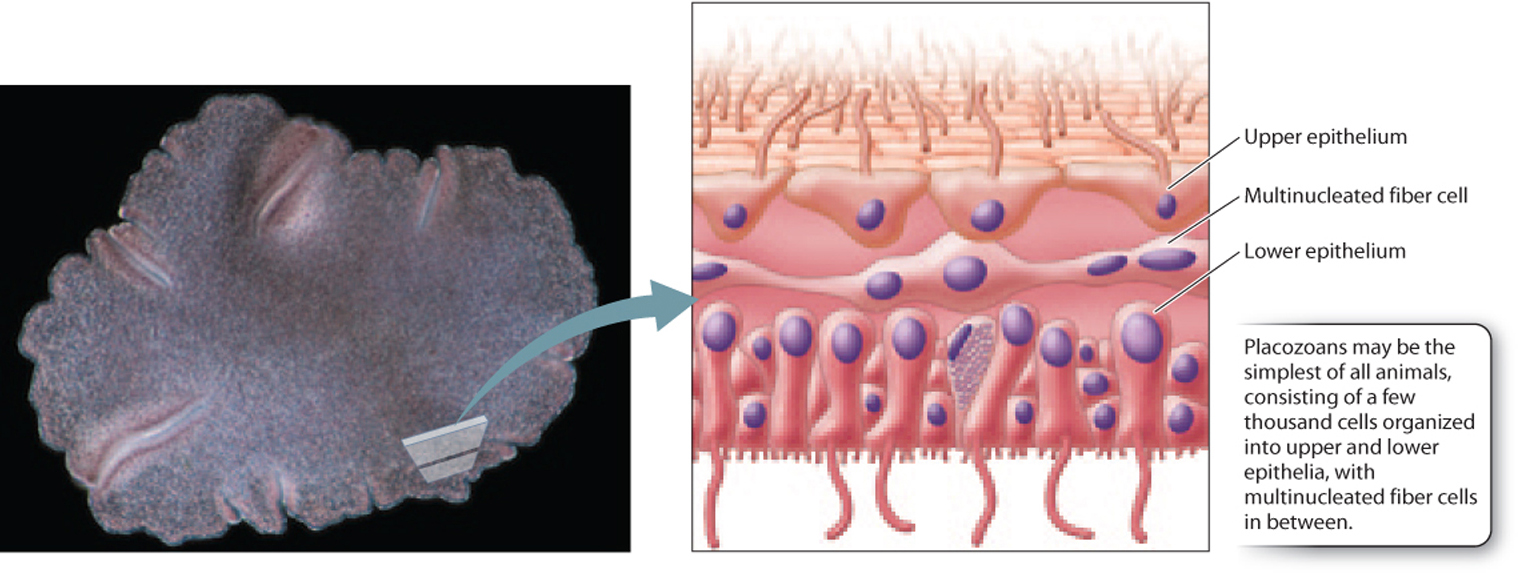
Despite their morphological simplicity, placozoans have a genome that contains many of the genes for transcription factors and signaling molecules that are present in cnidarians and bilaterian animals. What roles these genes play in placozoan biology remains unclear. A single species of placozoan, Trichoplax adhaerens, is known. First observed adhering to the inner wall of a marine aquarium, Trichoplax occurs widely in tropical and subtropical oceans. A second species was described in 1896 but has not been observed since that time. Fig. 44.13 shows one current view of the phylogenetic relationships among sponges and eumetazoans. It should be regarded as a set of hypotheses to be tested against new data rather than an unambiguously “correct” tree. See Table 44.1 for a list of all currently recognized animal phyla.
| Animal Phyla | |||
|---|---|---|---|
| PHYLUM | COMMON NAME | LIMB OF ANIMAL TREE | APPROXIMATE NUMBER OF DESCRIBED SPECIES |
| Acanthocephala | Thorny-headed worms | Lophotrochozoa | 1000 |
| Acoelomorpha | Acoel flatworms | Basal Bilateria (?) | 350 |
| Annelida | Segmented worms | Lophotrochozoa | 15,000 |
| Arthropoda | Arthropods | Ecdysozoa | 800,000—1,000,000 |
| Brachiopoda | Lamp shells | Lophotrochozoa | 350 |
| Bryozoa | Moss animals, sea mats | Lophotrochozoa | 5000 |
| Chaetognatha | Arrow worms | Protostomia | 100 |
| Chordata | Chordates | Deuterostomia | 50,000 |
| Cnidaria | Corals, jelly fish, etc. | Early branching Eumetazoa | 9000 |
| Ctenophora | Comb-jellies | Early branching Eumetazoa | 100—150 |
| Cycliophora | Symbion | Lophotrochozoa | At least 3 |
| Echinodermata | Echinoderms | Deuterostomia | 7000 |
| Echiura | Spoon worms | Lophotrochozoa | 150 |
| Entoprocta | Goblet worms | Lophotrochozoa | 150 |
| Gastrotricha | Hairy backs | Lophotrochozoa | 700 |
| Gnathostomulida | Jaw worms | Lophotrochozoa | 100 |
| Hemichordata | Acorn worms, pterobranchs | Deuterostomia | 90 |
| Kinorhyncha | Mud dragons | Ecdysozoa | 150 |
| Loricifera | Brush heads | Ecdysozoa | 120 |
| Micrognathozoa | Jaw animals | Lophotrochozoa | 1 |
| Mollusca | Mollusks | Lophotrochozoa | 80,000 |
| Nematoda | Roundworms | Ecdysozoa | 20,000 |
| Nematomorpha | Horsehair worms | Ecdysozoa | 320 |
| Nemertea | Ribbon worms | Lophotrochzoa | 1200 |
| Onychophora | Velvet worms | Ecdysozoa | 75 |
| Phoronida | Horseshoe worms | Lophotrochozoa | 20 |
| Placozoa | Trichoplax adhaerens | Early branching Eumetazoa | 1 |
| Platyhelminthes | Flatworms | Lophotrochozoa | 25,000 |
| Porifera | Sponges | Basal animals | 9000 |
| Priapulida | Priapulid worms | Ecdysozoa | 17 |
| Rhombozoa | — | Lophotrochozoa | 75 |
| Rotifera | Rotifers | Lophotrochozoa | 2000 |
| Sipuncula | Peanut worms | Lophotrochozoa | 350 |
| Tardigrada | Water bears | Ecdysozoa | More than 600 |
| Xenoturbellida | — | Deuterostomia | 2 |