44.3 BILATERIAN ANIMALS
The phyla discussed in the preceding section include about 20,000 species, a tiny fraction of all animal diversity. The final stop in this broad tour is the bilaterian branch, on which most animal species reside. Earlier, we pointed out that basic patterns of embryo formation divide bilaterians into two major groups, the Protostomia and the Deuterostomia (see Fig. 44.5). Molecular sequence data support the protostome–deuterostome groupings of bilaterian animals and show that the protostome animals can be further divided into two groups: the Lophotrochozoa (which include mollusks and annelid worms) and the Ecdysozoa (which include insects and other arthropods).
As noted above, bilaterian animals have bilateral symmetry and complex organs that develop from a triplobastic (three-germ layer) embryo. The anatomical complexity of bilaterian animals makes possible types of locomotion, feeding, gas exchange, behavior, and reproduction that are unknown in earlier branching groups. These capabilities underpin the remarkable diversity of bilaterian animals.
44.3.1 Lophotrochozoans make up nearly half of all animal phyla, including the diverse and ecologically important annelids and mollusks.
The Lophotrochozoa are a group of animals first recognized on the basis of unique molecular characters and now known to include several very different types of animal. On the one hand, there are the brachiopods, Bryozoa, and related phyla that have a tentacle-lined organ for filter feeding called a lophophore. Then, there are the mollusks, annelid worms, and their close relatives, characterized by a type of larva called a trochophore. The odd name “lophotrochozoan” is a contraction of the terms “lophophore” and “trochophore,” with “zoan” added to indicate that these organisms are animals (zoon is Greek for “animal”). The majority of lophotrochozoan species also have a distinctive form of spiral cleavage early in development.
The Lophotrochozoa contains 17 phyla, mostly small marine animals of limited diversity, but it also includes the diverse and ecologically important annelid worms and mollusks (Fig. 44.14). We’ll focus on these two phyla, which are closely related but have nonetheless evolved along quite different anatomical trajectories in the half billion years since their origins.
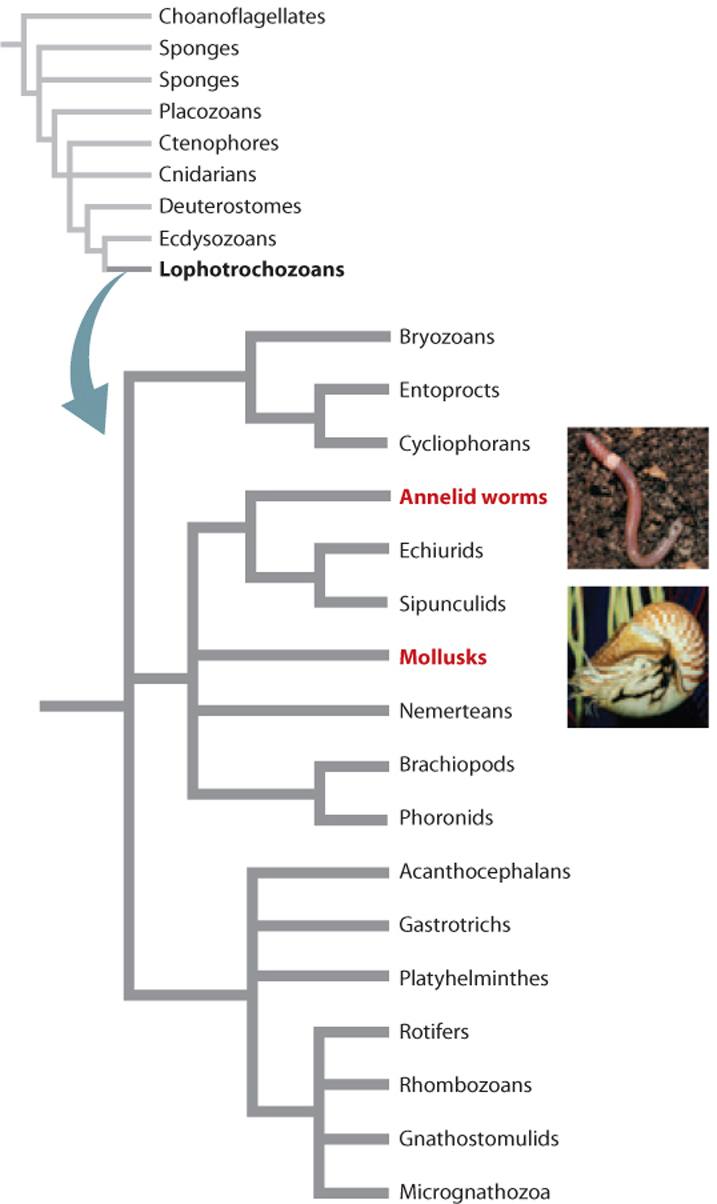
The annelids most familiar to us are the earthworms, commonly found in soil (Figs. 44.15a and 44.15b). Most of the 15,000 known annelid species, however, live in the oceans (Fig. 44.15c). All annelids have a cylindrical body with distinct segments, and they illustrate the advantages of a bilaterian body plan (see Fig. 44.2b). At one end, the head has a well-developed mouth and, internally, a cerebral ganglion (a collection of nerve cells) that connects to an extensive nervous system. A digestive system extends through the body from the head to an anus, with a sequence of specialized organs for crushing, then digesting, and finally excreting ingested food, much like the digestive system in our own bodies. Working together, these features enable annelids to move through their environment, actively searching for food and digesting it efficiently after ingestion.
Aquatic annelids have gill-like organs for gas exchange, but terrestrial earthworms exchange gases through their skin. In both cases, a closed circulatory system moves dissolved gases through the body. Annelids have waste-filtering organs called nephridia, gonads (repeated in most segments), and a fluid-filled coelom, or body cavity. Fluids in the coelom form a hydrostatic skeleton that works in coordination with paired muscles in each segment to direct movement.
Many annelids are predators that capture and ingest prey, but some, like earthworms, ingest sediment, digesting the organic matter it contains and excreting mineral particles. A few marine annelids have evolved tentacles that enable them to filter food particles from water, while leeches, a specialized group of freshwater annelids, attach themselves to vertebrates to suck out a meal of blood (Fig. 44.15d). And, remarkably, the meter-long vestimentiferan worms that live around hydrothermal vents in the oceans have given up ingestion altogether (Fig. 44.15e). Without mouths, these enormous worms gain nutrition from chemosynthetic bacteria that live within a collar of specialized tissue. Discovered in the 1970s, vestimentiferan worms were originally placed in their own phylum, but molecular sequence comparisons indicate that they are annelids.
Mollusks are the second major phylum of the Lophotrochozoa. Mollusks develop a distinctive larva called a trocophore that has a tuft of cilia at its top and additional cilia bands around its middle. Marine annelids develop from trochophores as well, and the presence of this unique form of larva in both phyla suggests a close evolutionary relationship between the two. This hypothesis is supported by molecular sequence comparisons. Nonetheless, mollusks and annelids share very few features of the adult body plan. Mollusks are distinguished by a unique structure called the mantle that plays a major role in breathing and excretion, and which forms their shells when present. In fact, mollusks by themselves exhibit a remarkable variety of forms and functions. More than 80,000 species of mollusks have been described, most of them gastropods.
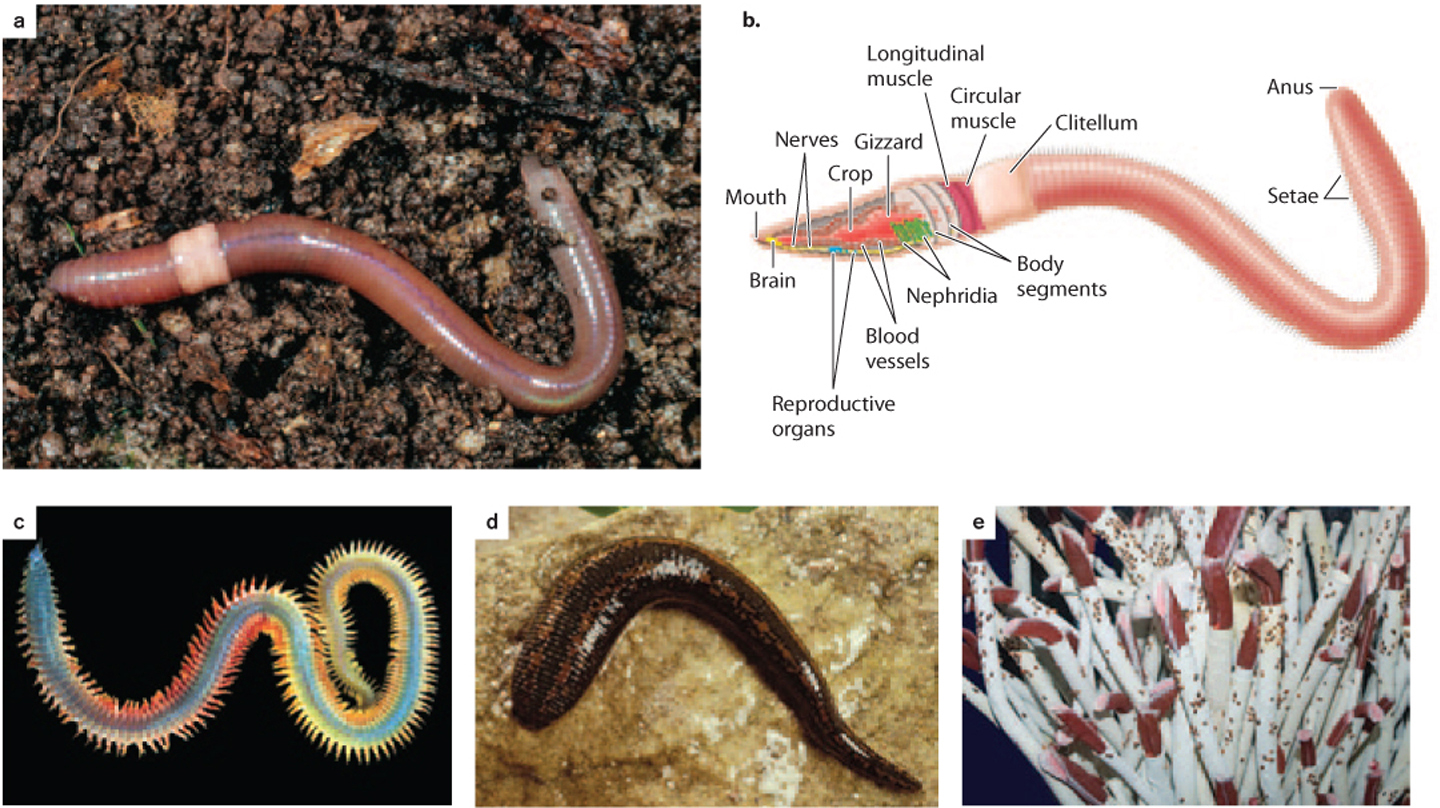
Gastropods—snails and slugs—provide an illuminating example of the mollusk body plan (Fig. 44.16). Gastropods have a head with a well-developed mouth that contains a toothlike structure called a radula for feeding. The mouth connects to a gut cavity that extends to an anus. Feather-like gills facilitate gas exchange, and a muscular foot is used for locomotion. A neural ganglion in the head coordinates a nervous system that extends through a body that also contains a well-developed circulatory system, gonads, and nephridia. Gastropods are coelomates, but the body cavity is generally reduced to small pouches that surround the heart and other organs. The outer surface of the body consists of the mantle. In many gastropods the mantle tissues secrete external skeletons of calcium carbonate, which form shells.
Some gastropods eat algae, but many are predators. About half occur in the ocean, and the other half are freshwater and terrestrial species that primarily feed on plants. In land snails and slugs, the only terrestrial mollusks, the gills have been lost, and gas exchange occurs in an internal cavity that has been modified to function as a lung.
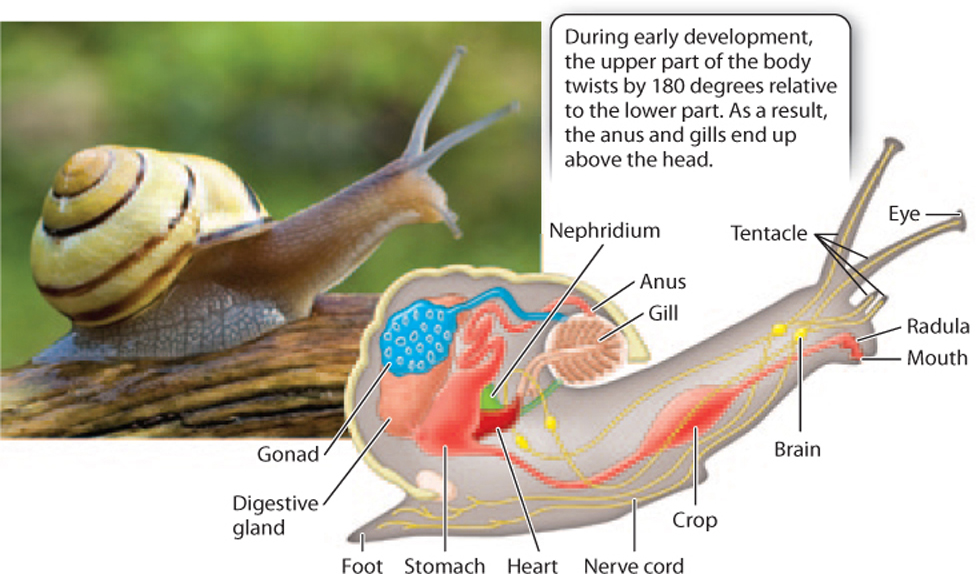
The second great class of mollusks is the cephalopods—about 700 species of squid, cuttlefish, octopus, and the chambered nautilus (Fig. 44.17). Cephalopods share a number of features with gastropods, including much of the internal anatomy, feather-like gills, and mantle. But they also have distinctive adaptations for their unique modes of life. Most obviously, cephalopods have muscular tentacles that capture prey and sense the environment. Surprisingly, these leglike appendages are found on the head region rather than on the sides of the body as in other animals.
Cephalopods are superb swimmers, able to dart through the water by means of a jet propulsion system that forces water through mantle tissue that is fused to form a siphon. It is this combination of tentacles and rapid locomotion that makes cephalopods important predators in the oceans. Moreover, cephalopods have exceptional eyesight and exhibit the most complex behavior of any invertebrate animals, able to learn visual patterns and solve puzzles to gain food. The chambered nautilus secretes a coiled shell of calcium carbonate (Figs. 44.17a and 44.17b), but in other cephalopods, like squid and octopus, the shell is reduced or absent (Fig. 44.17c). In oceans of the Mesozoic Era (252–65 million years ago), however, shelled cephalopods called ammonites were among the most abundant and diverse predators.
A third major group of mollusks, the bivalves, includes the familiar clams, oysters, and mussels. Once again, these animals have anatomical features that point to their close relationship to snails and squids, but also have distinctive features that set them apart (Fig. 44.18). Notably, bivalves have (literally) lost their heads. As their name implies, bivalves have evolved a skeleton in which two hard shells called valves are connected by a flexible hinge.
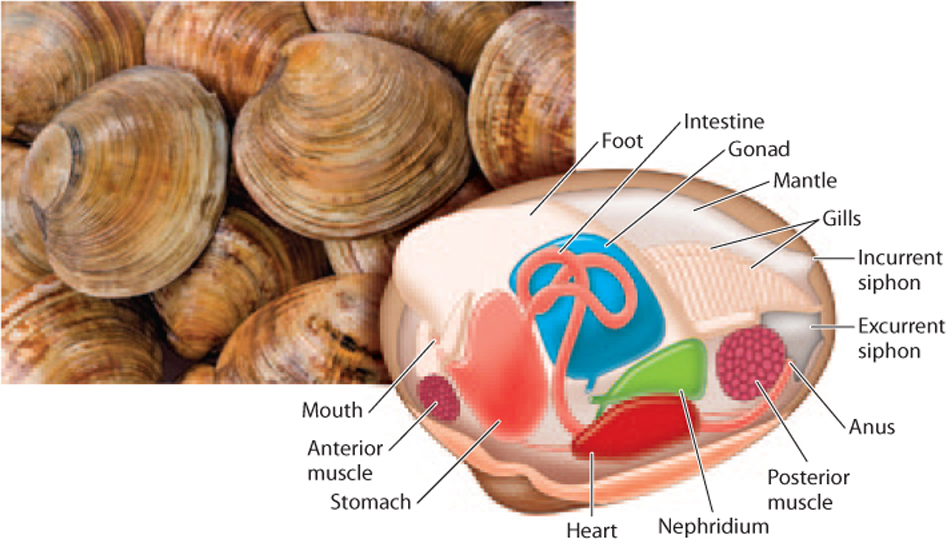
Most bivalves obtain food by filtering particles from seawater. However, many bivalves live within marine sediments, burrowing into sand or mud with their muscular foot. How can bivalves filter seawater within the sediment? Once again, this group of mollusks has a modified mantle that allows a new function. Flaps of mantle tissue have fused to form a pair of siphons that extend upward from the bivalve’s body to the surface of the seafloor above it. One siphon draws water containing food and oxygen into the body. The second siphon then returns water and waste materials to the environment. About 2000 species of bivalves have been described, mostly from the oceans, but also many from freshwater.
Quick Check 3
From the shared traits of present-day snails, squids, and clams, what features do you think the common ancestor of all three had?
44.3.2 Ecdysozoans include arthropods, the most diverse animals.
The second major group of protostome animals is the Ecdysozoa (Fig. 44.19), which get their name from the process of ecdysis, or molting. All ecdysozoans secrete a cuticle made of protein that covers their bodies. This tough cuticle is like a suit of flexible, lightweight armor, protecting bodies from injury and physical challenges of the environment such as drying. This “armor” has a special property: The hard cuticle can be used to form appendages that function as tools, weapons, or even wings. Like a suit of armor, the cuticle is always a perfect fit for the wearer but does not stretch, and so it must be exchanged episodically during growth for a larger size to fit a larger, growing body. Ecdysozoans, then, are animals that molt their external cuticle during growth, producing a very soft, larger replacement underneath that soon hardens into a new protective covering.
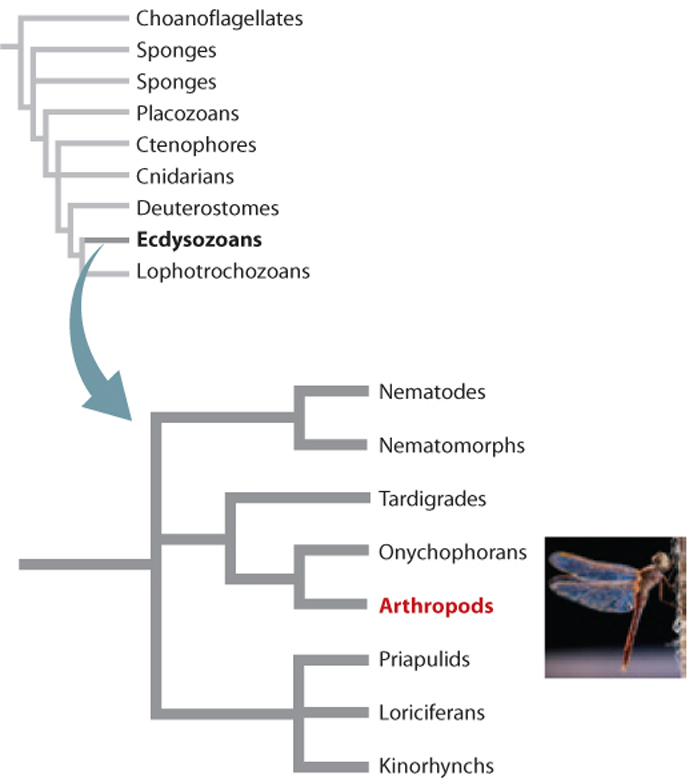
The Ecdysozoa include eight phyla, but one dwarfs the rest in anatomical complexity, diversity, and ecological importance. As noted earlier, the Arthropoda, including insects, contains more than half of all known animal species. What features have led to the remarkable evolutionary success of this branch?
Perhaps the most obvious feature of arthropods is the one for which they are named: jointed legs (arthro means “jointed,” and pod means “foot” or “leg”). The jointed leg is the most versatile part of the arthropod body; it has been modified through evolution into structures that function as paddles, spears, stilts, pincers, needles, hammers, and more. The jointed leg allowed arthropods to colonize land by providing a multipoint, independent suspension—much like that of an all-terrain vehicle—able to support a relatively large body and move over the most uneven ground. So well adapted for locomotion is the arthropod leg that NASA engineers are using it as a model for planetary exploration vehicles. Researchers have conducted many studies to understand how this one type of limb could give rise to so many diverse structures with different functions (Fig. 44.20).
FIG. 44.20How did the diverse feeding appendages of arthropods arise?
BACKGROUND Arthropod species diversity is matched by their diversity of form and function, challenging scientists to recognize and relate the equivalent parts of the very different front ends of, for example, spiders, crabs, and centipedes.
HYPOTHESIS Researchers hypothesized that the diverse front appendages of the different classes of arthropods develop from similar limb buds clearly visible in developing embryos, much as the limb buds leading to bird wings, whale flippers, and human arms are present in vertebrate embryos.
EXPERIMENT The early stages of arthropod embryos have a definite head region and several body segments, each with a pair of appendages. Hox genes specify the identity of each body segment (Chapter 20). The expression patterns of different Hox genes can be visualized by means of labeled probes on whole embryos. In this way, the development of appendages on the same segment in different types of arthropod can be compared.
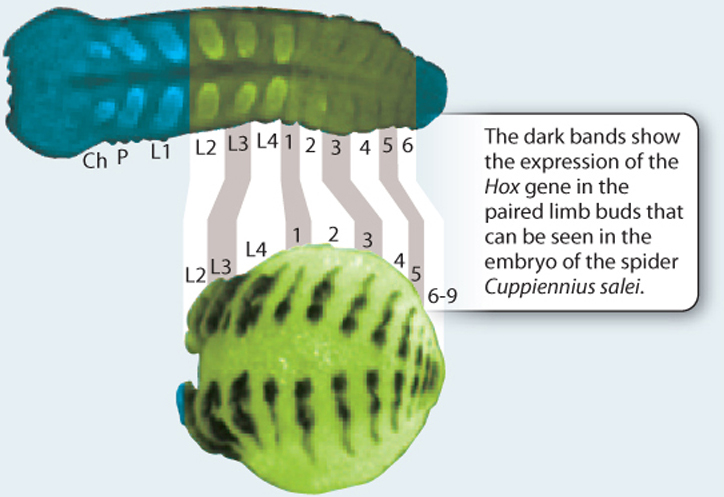
RESULTS Study of the embryos revealed that, for example, the jawlike mandibles of insects and crustaceans form from appendages on the same segments of the developing body, whereas this segment on spiders and scorpions bears a pair of leglike feelers. Behind the mandibles are two pairs of feelers that are represented by legs in spiders and scorpions. On the other hand, the fangs of spiders and their counterparts in scorpions arise from a segment closer to the front end than the mandibles.
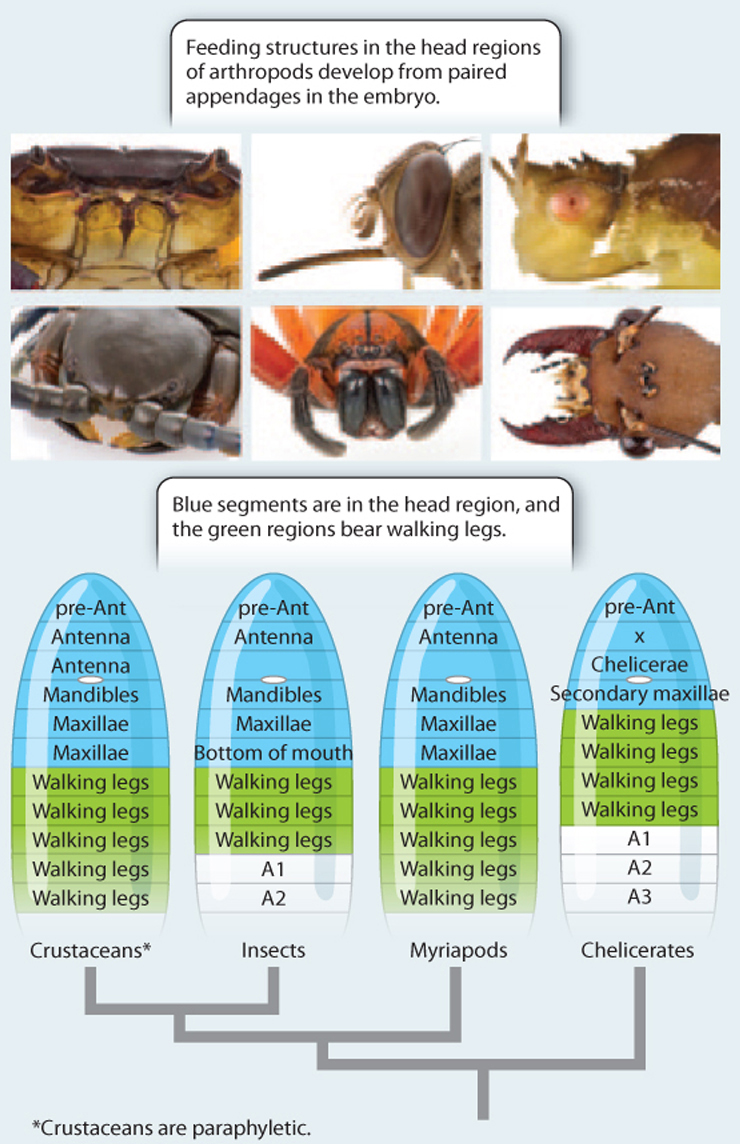
CONCLUSIONS The diverse feeding structures of arthropods arise from limb buds in the first segments of the developing embryo. The mandibles of crustaceans and insects arise from the same body segment and indicate the common ancestry of these animals, while spiders, scorpions, and their relatives are more distantly related. Evolution has modified simple legs into the remarkable diversity of forms, enabling structures as specialized as a spider’s fangs and the grinding mandibles of crabs.
FOLLOW-UP WORK There has been recent controversy over the front appendages of sea spiders and whether or not they are equivalent to the great appendage on some Cambrian arthropod fossils.
SOURCE Damen, W. G. M., M. Hausdorf, E.-A. Seyfarth, and D. Tautz. 1998. “A Conserved Mode of Head Segmentation in Arthropod Revealed by the Expression Pattern of HOX Genes in a Spider.” Proceedings of the National Academy of Sciences USA 95:10665–10670.
The arthropods’ other defining characteristic is the material that forms their hard external skeleton: a strong, lightweight and nearly indestructible polysaccharide called chitin. Chitin, you may recall from Chapter 34, also forms the wall of fungal cells. Next to the cellulose that forms the wall of plant cells, chitin may be the most abundant biomolecule on Earth.
There are four main groups of arthropods (Fig. 44.21), of which the most diverse by far is the insects. The other three are chelicerates, including spiders, scorpions, and their relatives; myriapods, including centipedes and millipedes; and crustaceans, including lobsters, shrimp, crabs, and their relatives.
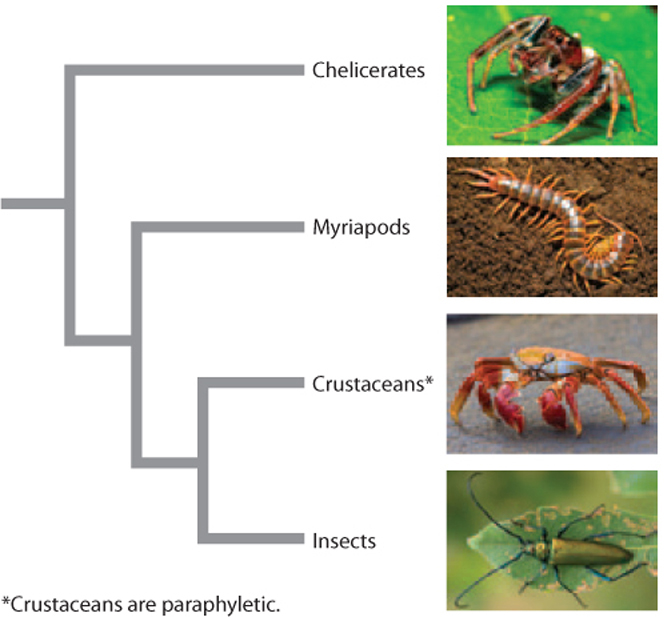
Chelicerates, named for the pincer-like claws called chelicerae, are the only arthropods that lack antennae. The chelicerates are mostly carnivores, except for plant-eating mites. Scorpions and spiders are known for their venoms, used to subdue prey. In spiders, the venom is injected through chelicerae modified to form fanglike appendages, while in scorpions the venom is injected through the curved tail segment of their abdomen. Other familiar chelicerates include the harvestmen (often called daddy long-legs in the United States), ticks, and horseshoe crabs. Giant sea scorpions called eurypterids, up to 2 m long, were dominant predators in warm shallow seas 450–250 million years ago. The chelicerates were among the very first animals to invade land, about 400 million years ago, aided by their protective cuticle and supported by their arrays of jointed legs.
Myriapods are named for their many pairs of legs, which make them easy to recognize. Centipedes (“hundred legs”) may have up to 300 pairs of legs and are fast-moving predators with front legs modified into venomous, fanglike organs. Millipedes (“thousand legs”) may have several hundred pairs of legs (though never a thousand) and are slow-moving herbivores and detritivores (eaters of dead organisms). Millipedes often have glands lining their long bodies that produce a substance that contains cyanide, which they secrete to defend against predators.
Crustaceans also have distinctive appendages, notably their branched legs. They navigate their world with the aid of two pairs of highly sensory antennae, the larger of which has two branches. The unique crustacean larva, called a nauplius, swims with the aid of the smaller antenna and has a single eye that typically disappears in the adults. In the sea, these versatile arthropods fill many of the ecological roles that insects play on land, eating plants, other animals, or detritus. The tiny shrimplike plankton known as krill occur in vast numbers in polar seas, supporting ecosystems of fish, birds, and mammals such as whales. One Antarctic krill species is estimated to total over 500 million tons of biomass, more than the global total for humans. Lobsters and crabs are largely detritivores, scouring the ocean bottoms for dead and dying animals. Some crustaceans are even parasites, attaching themselves to the skin or inside the mouths of fish or sea mammals in order to feed.
Insects were the first animals to evolve wings, nearly 350 million years ago, and modern-day mayflies and dragonflies still bear structural similarities to these first fliers. The earliest branching members of this group, the silverfish and rockhoppers, are referred to as primitively wingless because they evolved before the origin of wings. Some other insects, like fleas, have lost wings that were present in their common ancestor, presumably because their parasitic lifestyles are better served by jumping than flying. Like all arthropods, insects repeatedly shed their exoskeleton as they grow into adulthood. No insect can molt wings, however, and so only adults can fly.
Within the insects, the main evolutionary dividing line is between those that undergo metamorphosis, a major change in form from one developmental stage to another, and those such as grasshoppers and water bugs that do not. In insects that do not undergo metamorphosis, the main change in body form involves the appearance of wings, though some, like dragonflies, may also change the form of their legs or eyes as they leave the aquatic environment.
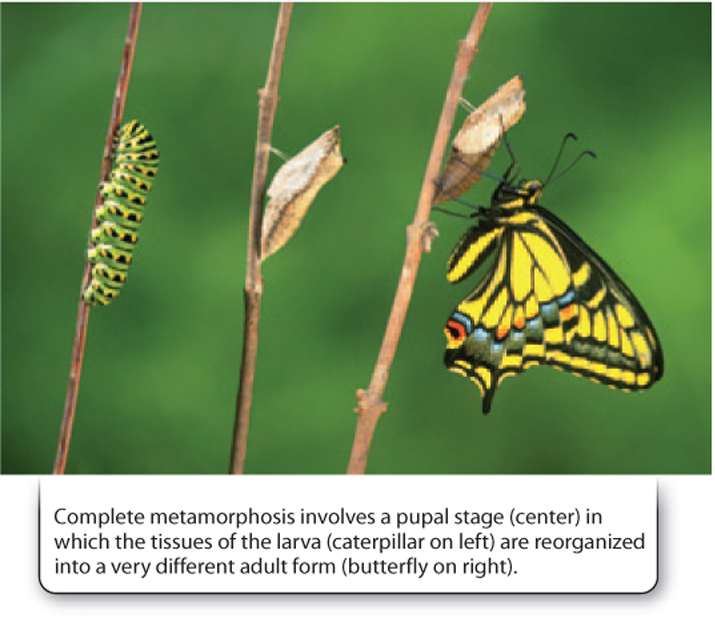
However, for those insects undergoing metamorphosis, the body changes from a wormlike larva specialized for feeding to a stage called a pupa (Fig. 44.22). During the pupa stage, the body tissues undergo a remarkable transformation from the relatively simple larva to a very different looking adult such as a fly, butterfly, wasp, or beetle, usually specialized for reproduction. Insects undergo a nearly complete dissolution of their muscles and other body parts, and so require the immobile, coffin-like pupa in which to transform their bodies from something as simple as a maggot, which lacks even an obvious head or tail, into an animal as sophisticated as a fly, able to walk on water, hover motionless in front of a flower, or fly at 60 mph. This evolutionary step has little parallel elsewhere in life and is one of the keys to insect diversification.
Unlike other terrestrial arthropods, all insects, whether or not they undergo metamorphosis, have highly specialized eggshells that can withstand desiccation while still allowing gas exchange. This shell allows insect eggs to survive in dry settings such as the surfaces of leaves, and so they can occupy habitats that are difficult for other terrestrial arthropods to access.
Insects have evolved one other critical adaptation for life on land. Aquatic arthropods obtain oxygen through gills, which don’t work well in air because their large surface area dries out. Some terrestrial chelicerates have evolved simple lungs, called book lungs: modified appendages filled with hemolymph (blood) that have a large surface area for gas exchange, but are kept moist inside a protective pocket. Some crabs are able to make extended forays on land by keeping their gills moist underneath their carapace. In contrast, insects exchange gases through small pores in their exoskeletons called spiracles (see Fig. 39.4b). The spiracles connect to an internal system of tubes, the tracheae, that direct oxygen to and remove carbon dioxide from respiring tissues, much like air exchange ducts in a building.
Insects are extraordinarily diverse, with approximately 1 million described species making up 80% or more of all known animal species. Their success may be a function of the three adaptations that allow them to live in diverse habitats: dessication-resistant eggs, wings, and metamorphosis. The ability of insect eggs to resist drying out means that they can be placed on virtually any surface. Their wings allow them to reach habitats and feed in ways other animals cannot. Metamorphosis allows them to have two bodies in their life cycles: a larval form specialized for feeding and an adult form specialized for mating and reproduction. These three adaptations enabled insects to use flowering plants directly as food. In turn, many insects pollinate plants, and so pollinating insects are in part responsible for flowering plant diversity (Chapter 33).
44.3.3 Deuterostomes include humans and other chordates, but also acorn worms and sea stars.
In a classification recognized long ago by shared features of larval development, and strongly supported by molecular sequence data, the Deuterostomia (Fig. 44.23) include three major phyla: Chordata (vertebrates and closely related invertebrate animals such as sea squirts), Hemichordata (acorn worms), and Echinodermata (sea urchins and sea stars).
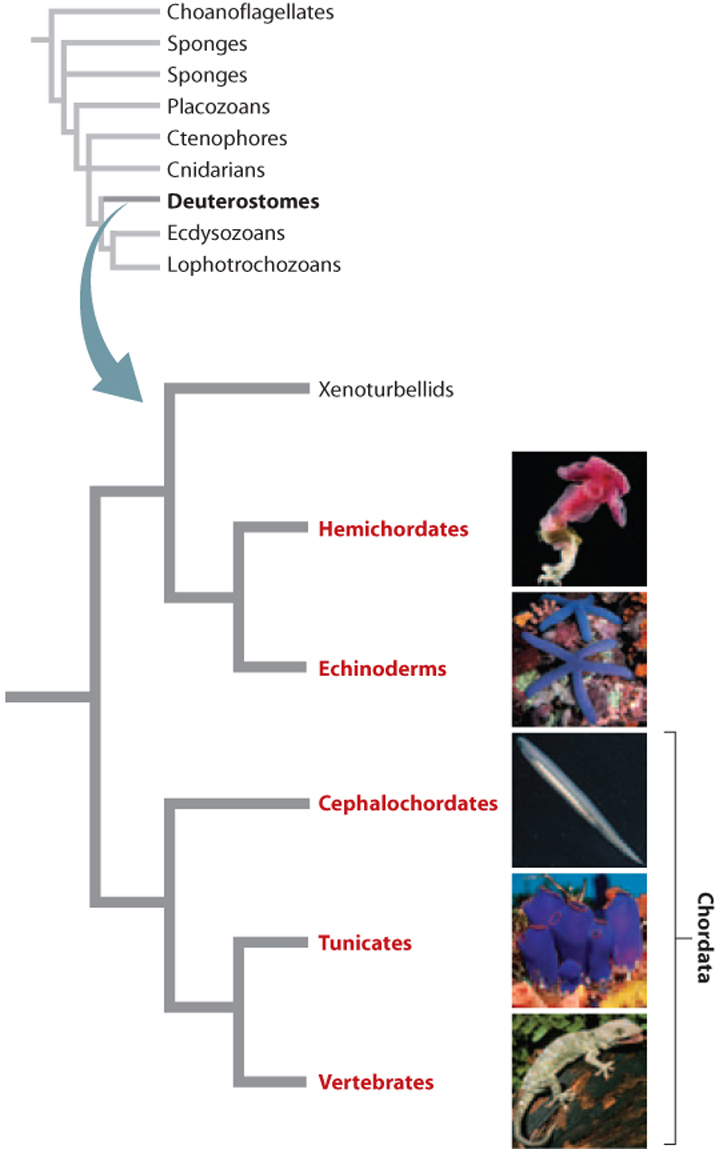
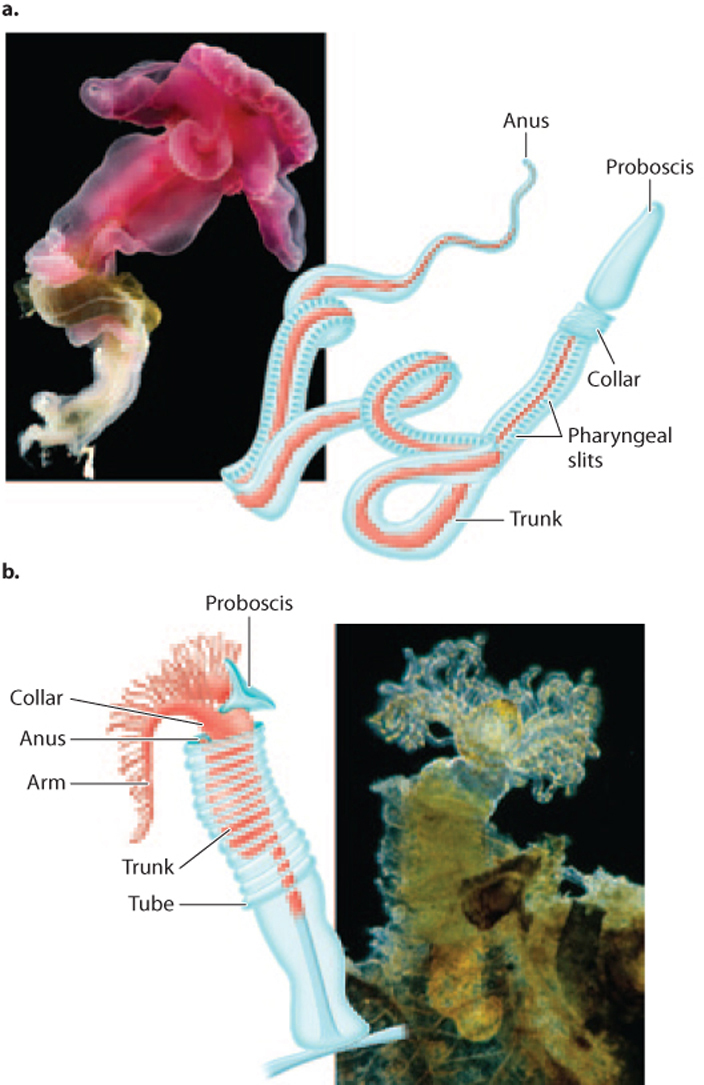
Let’s begin with the deuterostomes most distantly related to the vertebrates, the hemichordates and echinoderms. Hemichordates include acorn worms, about 75 species of wormlike animals that move through seafloor sediments in search of food particles. Pterobranchs, also hemichordates, consist of about a dozen species of animals that attach to the seafloor and use tentacles to filter food from seawater (Fig. 44.24). Hemichordates all have a mouth on an elongate protuberance called a proboscis that connects to the digestive tract by a tube called the pharynx, which contains a number of vertical openings called pharyngeal slits separated by stiff rods of protein. They also have a dorsal nerve cord. Additional characters that relate hemichordates to other deuterostomes are apparent in embryological and larval development. Nonetheless, living hemichordates have body plans quite distinct from those of chordates and echinoderms.
Another major group of deuterostomes is the echinoderms, among the most distinctive of all animal phyla. Echinoderms include sea stars and sea urchins (including sand dollars), as well as brittle stars, sea cucumbers, and sea lilies—all of which share a unique fivefold symmetry on top of their basic bilaterian organization (Fig. 44.25). Echinoderms also form distinctive skeletons made of interlocking plates of porous calcite, a form of calcium carbonate. The third unique feature of echinoderms is the water vascular system, a series of fluid-filled canals that permits bulk transport of oxygen and nutrients. Tube feet, small projections of the water vascular system that extend outward from the body surface, facilitate locomotion, sensory perception, food capture, and gas exchange. About 7000 echinoderm species reside in present-day oceans, but the fossil record suggests that this phylum was more diverse in the past.
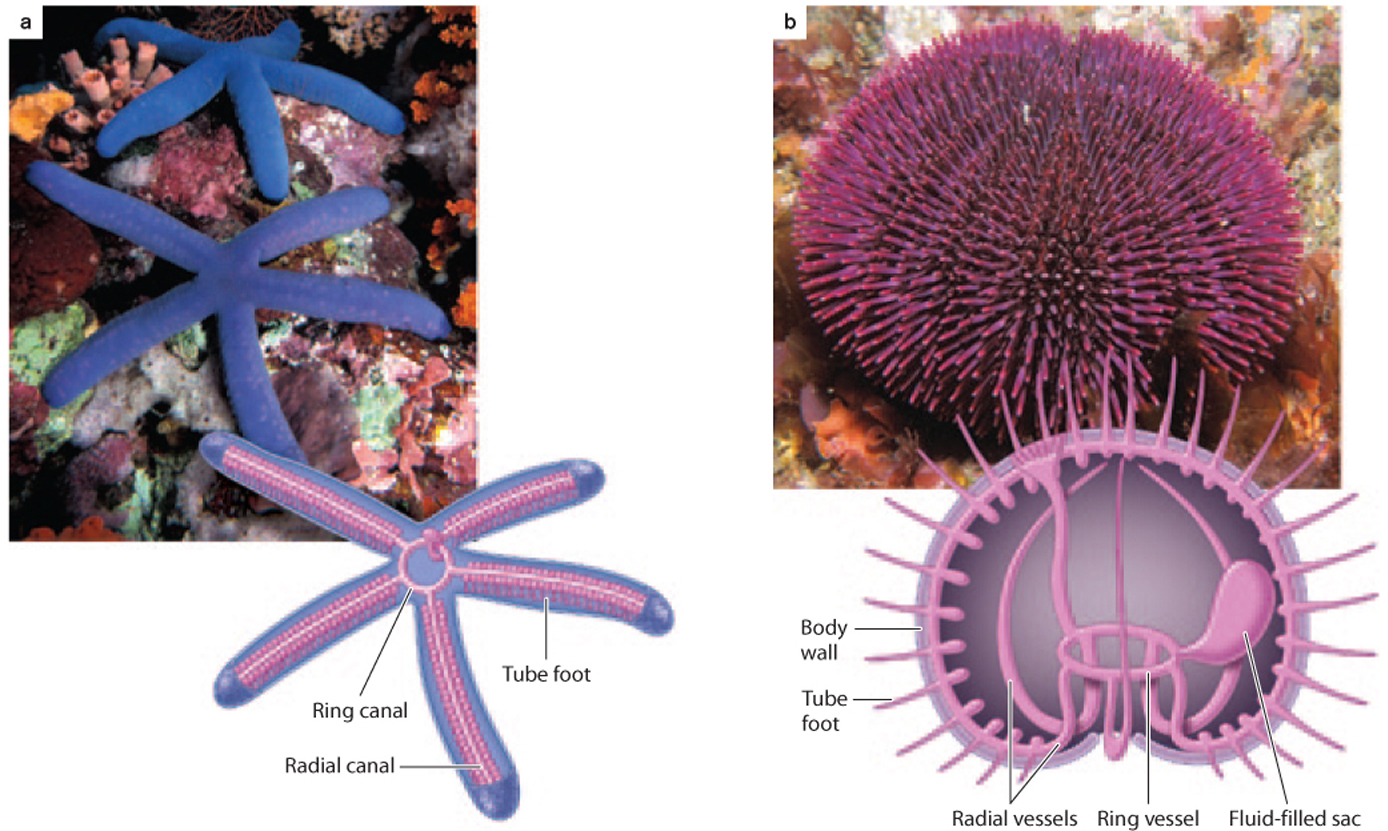
Zoologists have long known that echinoderm and hemichordate larvae share many features. Molecular sequence data now confirm that echinoderms are the closest relatives of hemichordates, with chordates a sister group to the group containing echinoderms and hemichordates (see Fig. 44.23).
44.3.4 Chordates include vertebrates, cephalochordates, and tunicates.
The other great branch of the deuterostome tree is the chordates, the phylum that includes vertebrate animals. Within the phylum Chordata, there are three subphyla: the cephalochordates, the tunicates, and the vertebrates (also called craniates). Like hemichordates, chordates all have a pharynx with pharyngeal slits (well illustrated by the invertebrate chordate amphioxus in Fig. 44.26). In fish, these pharyngeal slits form the gills, but in terrestrial animals like humans, these slits can be seen only in developing embryos. The notochord, a stiff rod of collagen and other proteins, runs along the back, providing support for the axis in some chordates. In vertebrates, the notochord is apparent only during early embryogenesis and is replaced functionally by the development of a vertebral column. Also forming during early development is the neural tube, a cylinder of embryological tissue that develops into a dorsal nerve cord. Body musculature is organized into a series of segments called myotomes. Chordates have a tail (reduced but still internally present in humans and other apes) that extends posterior to the anus and muscularized appendages that include the fins of fish and legs of terrestrial vertebrates.
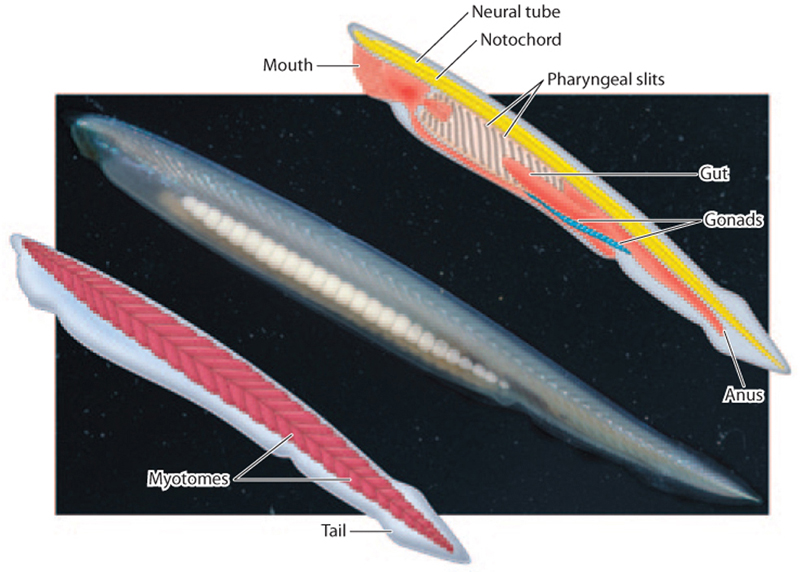
Amphioxus (Fig. 44.26) belongs to the cephalochordates. These animals share key features of body organization with vertebrates but lack a well-developed brain and eyes, have no lateral appendages, and do not have a mineralized skeleton. Nonetheless, their many similarities to vertebrates suggest they are closely related, and molecular sequence comparisons confirm this hypothesis.
The tunicates also share key features of the chordate body plan during early development, but they have a unique adult form (Fig. 44.27). Tunicates include about 3000 species of filter-feeding marine animals, such as sea squirts anchored to the seafloor and salps that float in the sea. The adult tunicate body is a basket-like structure that is highly modified for filter-feeding. It might be mistaken for a sponge, but its complex internal structure shows that it lies far from sponges on the animal tree. The tunicate’s body wall, or tunic, has a siphon-like mouth at one end that draws water through an expanded pharynx that captures food particles and exchanges gases. Water and wastes are expelled through an anal siphon. In the adult tunicate, the only obvious similarities to other chordates are the pharynx and its pharyngeal slits. Larval tunicates, however, resemble other chordates like fish or tadpoles with a more typical chordate body plan, including a notochord, neural tube, and a long tail with muscles arranged in myotomes.
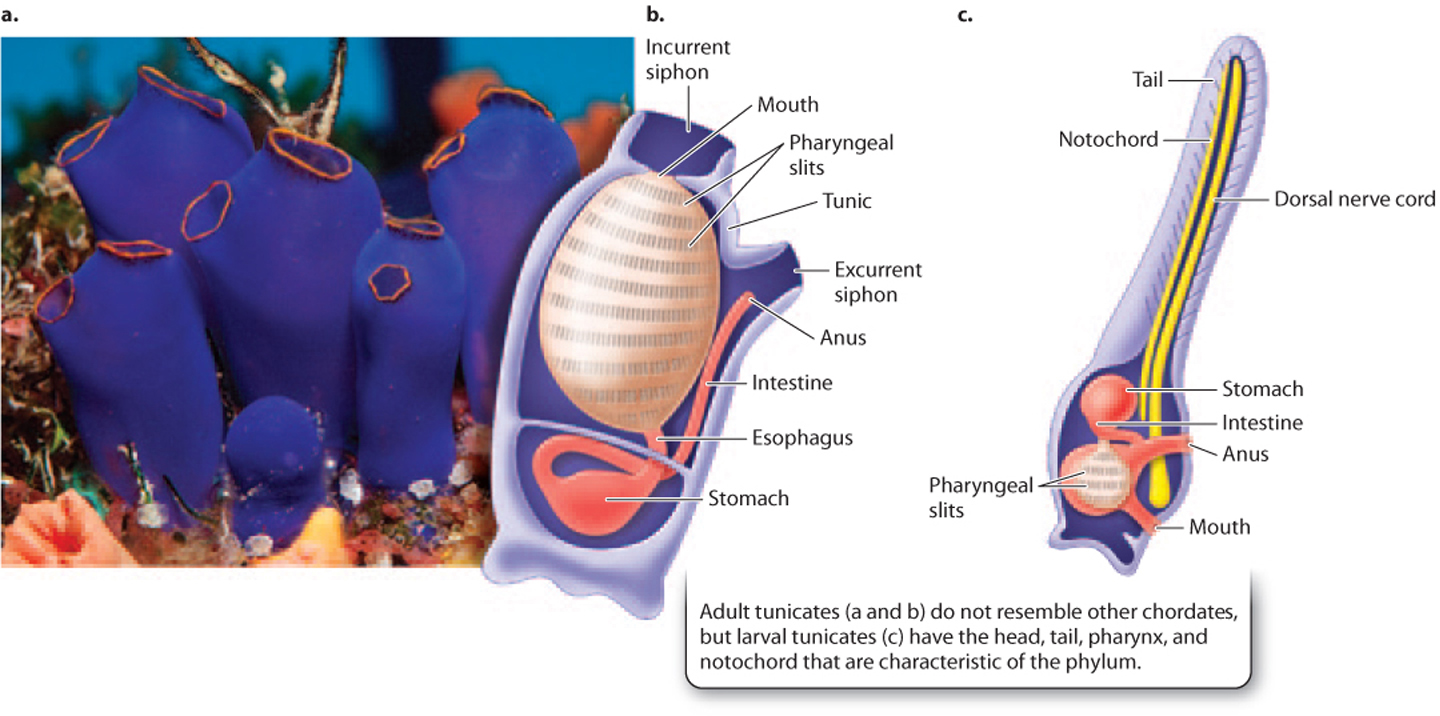
For many years, zoologists considered cephalochordates to be the closest relatives of vertebrate animals, but molecular data now suggest that, in fact, the tunicates are our closest invertebrate relatives. This means that the common ancestor of tunicates, amphioxus, and vertebrate animals was a small animal much like amphioxus in organization. After the divergence of the three major groups of chordates, however, the tunicates’ adaptation for filter-feeding led to the unique anatomy they have today.
Quick Check 4
You do not have a notochord, a tail, or gill slits. How do we know that you are a chordate?