Chapter 1. Learning Progression Guide
Overview
Overview

Assessment is woven into every aspect of How Life Works.
- Learning Progressions include reading comprehension questions, in-class activities, post-class questions, and exam questions – all properly aligned with the text’s core concepts. Most questions from the progressions can be used to suit an instructor's needs. Used as a progression, the questions provide a connected learning path for students and a suggested teaching path for instructors.
- Basic Knowledge Questions are lower order questions that have been carefully aligned with the text’s core concepts. They can be used in concert with the assessment Progressions to develop well-rounded learning path.
- Every section of the e-book contains embedded free-response and multiple-choice Quick Check questions. Assign e-book sections to your students to ensure they read and master the textbook material.
- Animation, Simulation, and Visual Synthesis activities all include specially-written questions that students can answer to demonstrate that they've completed and understood the content.
- The Chapter Summary includes free response Self Assessment questions. If you assign the Chapter Summary, students will be required to provide an answer to at least one of these questions, and can view answers for the rest.
This instructor-only Learning Progression Guide shows the Learning Progression questions, with suggestions for how they might be used by an instructor—pre-class, in-class, post-class, and/or on exams. See the "BKQs" section for a full listing of all Basic Knowledge Questions. Finally, see the Pre-Made Assessments section to see how Learning Progression and Basic Knowledge questions can populate pre-made Pre-Class and, Homework assignments as well as an exam. Questions designed specifically for use in class can be downloaded by the instructor.
Progression 4.1
Learning Progression 4.1
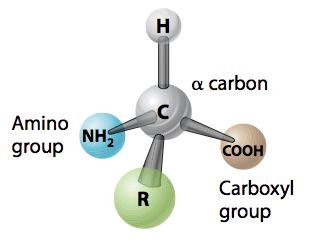
Students should be able to answer the questions in this activity if they've mastered the following Core Concept:
- 4.1 Proteins are linear polymers of amino acids that form three-dimensional structures with specific functions.
Progression Question 4.1.1A
1.
The structural formula below is of two amino acids joined together to form a dipeptide. The carbons in this dipeptide are numbered 1 through 4. Use this formula to answer the question below.
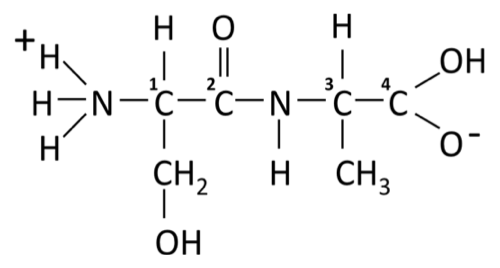
Which of the carbons is involved in a peptide bond?
| A. |
| B. |
| C. |
| D. |
- Figure 4.1
- Figure 4.2
- Figure 4.3
This question engages students with the structure of amino acids, peptide bonds, polypeptides, functional groups.
| Question Notes | |||||||
Core Concept(s): 4.1, 4.2 Level: LOC Type(s): MCQ, Small group discussion | Connections:
| Guidance: This question engages students with the structure of amino acids, peptide bonds, polypeptides, functional groups. | Uses:
| ||||
Free response question: Q: Draw two amino acids linked together by a covalent bond (that is, draw a dipeptide). Circle the peptide bond in your illustration. Label the alpha carbon atom(s), the hyhdrophobic side chain, the carboxyl group, and the amino group. A: | |||||||
Progression Question 4.1.1B
2.
The structural formula below is of two amino acids joined together to form a dipeptide. The carbons in this dipeptide are numbered 1 through 4. Use this formula to answer the question below.

Which of the carbons are alpha carbons?
| A. |
| B. |
| C. |
| D. |
| E. |
| F. |
- Figure 4.1
- Figure 4.2
- Figure 4.3f
This question engages students with the structure of amino acids, peptide bonds, polypeptides, functional groups.
| Question Notes | |||||||
Core Concept(s): 4.1, 4.2 Level: LOC Type(s): MCQ, Small group discussion | Connections:
| Guidance: This question engages students with the structure of amino acids, peptide bonds, polypeptides, functional groups. | Uses:
| ||||
Free response question: Q: Draw two amino acids linked together by a covalent bond (that is, draw a dipeptide). Circle the peptide bond in your illustration. Label the alpha carbon atom(s), the hyhdrophobic side chain, the carboxyl group, and the amino group. A: | |||||||
Progression Question 4.1.1C
3.
The structural formula below is of two amino acids joined together to form a dipeptide. The carbons in this dipeptide are numbered 1 through 4. Use this formula to answer the question below.

Which of the carbons is attached to a hydrophobic side chain?
| A. |
| B. |
| C. |
| D. |
- Figure 4.1
- Figure 4.2
- Figure 4.3
This question engages students with the structure of amino acids, peptide bonds, polypeptides, functional groups.
| Question Notes | |||||||
Core Concept(s): 4.1, 4.2 Level: LOC Type(s): MCQ, Small group discussion | Connections:
| Guidance: This question engages students with the structure of amino acids, peptide bonds, polypeptides, functional groups. | Uses:
| ||||
Free response question: Q: Draw two amino acids linked together by a covalent bond (that is, draw a dipeptide). Circle the peptide bond in your illustration. Label the alpha carbon atom(s), the hyhdrophobic side chain, the carboxyl group, and the amino group. A: | |||||||
Progression Question 4.1.1D
4.
The structural formula below is of two amino acids joined together to form a dipeptide. The carbons in this dipeptide are numbered 1 through 4. Use this formula to answer the question below.

Which of the carbons is attached to a carboxyl group?
| A. |
| B. |
| C. |
| D. |
- Figure 4.1
- Figure 4.2
- Figure 4.3
This question engages students with the structure of amino acids, peptide bonds, polypeptides, functional groups.
| Question Notes | |||||||
Core Concept(s): 4.1, 4.2 Level: LOC Type(s): MCQ, Small group discussion | Connections:
| Guidance: This question engages students with the structure of amino acids, peptide bonds, polypeptides, functional groups. | Uses:
| ||||
Free response question: Q: Draw two amino acids linked together by a covalent bond (that is, draw a dipeptide). Circle the peptide bond in your illustration. Label the alpha carbon atom(s), the hyhdrophobic side chain, the carboxyl group, and the amino group. A: | |||||||
Progression Question 4.1.2
- Figure 4.3
- Core Concept 2.5
This question engages students with the structure of amino acids and polypeptides, and reinforces the importance of condensation reactions in the synthesis of macromolecules.
5.
How many water molecules would be produced in making a polypeptide that is 14 amino acids long?
| A. |
| B. |
| C. |
| D. |
| Question Notes | |||||||
Core Concept(s): 4.1 Level: LOC Type(s): MCQ, Small group discussion | Connections:
| Guidance: This question engages students with the structure of amino acids and polypeptides, and reinforces the importance of condensation reactions in the synthesis of macromolecules. | Uses:
| ||||
Free response question: Q: How many water molecules would be produced in making a polypeptide that is fourteen amino acids long? A: Thirteen water molecules are formed by the thirteen condensation reactions that occur sequentially to join the fourteen amino acids. | |||||||
Progression Question 4.1.3A
- Figure 4.3
- Figure 4.4
- Figure 4.6
- Figure 4.7
- Figure 4.10
Using “real world” language of science, this question reinforces the importance of the different bonds that stabilize the four levels of protein structure.
6.
The three-dimensional shape of a protein is determined by the primary, secondary, tertiary, and in many cases, the quaternary structure of the protein. The following are sentences taken from scientific articles on protein structure. For each of the statements, choose the level of protein structure that applies best.
"Hydrogen bonds between peptide backbone components form a distinct helical structure."
| A. |
| B. |
| C. |
| D. |
| Question Notes | |||||||
Core Concept(s): 4.1 Level: HOC Type(s): MCQ, Small group discussion | Connections:
| Guidance: Using “real world” language of science, this question reinforces the importance of the different bonds that stabilize the four levels of protein structure. | Uses:
| ||||
Free response question: Q: The three dimensional shape of a protein is determined by the primary, secondary, tertiary, and in many cases, the quaternary structure of the protein. The sentences below are from scientific articles on protein structure. For each of the sentences, indicate the level of protein structure (primary, secondary, tertiary, or quaternary) that applies best.
(Correct answers are indicated in parentheses) | |||||||
Progression Question 4.1.3B
- Figure 4.3
- Figure 4.4
- Figure 4.6
- Figure 4.7
- Figure 4.10
Using “real world” language of science, this question reinforces the importance of the different bonds that stabilize the four levels of protein structure.
7.
The three-dimensional shape of a protein is determined by the primary, secondary, tertiary, and in many cases, the quaternary structure of the protein. The following are sentences taken from scientific articles on protein structure. For each of the statements, choose the level of protein structure that applies best.
"Hydrogen bonds between peptide 'backbone' amino groups and carboxyl groups of one polypeptide and the R-groups of another polypeptide, contribute to the overall function of the protein."
| A. |
| B. |
| C. |
| D. |
| Question Notes | |||||||
Core Concept(s): 4.1 Level: HOC Type(s): MCQ, Small group discussion | Connections:
| Guidance: Using “real world” language of science, this question reinforces the importance of the different bonds that stabilize the four levels of protein structure. | Uses:
| ||||
Free response question: Q: The three dimensional shape of a protein is determined by the primary, secondary, tertiary, and in many cases, the quaternary structure of the protein. The sentences below are from scientific articles on protein structure. For each of the sentences, indicate the level of protein structure (primary, secondary, tertiary, or quaternary) that applies best.
(Correct answers are indicated in parentheses) | |||||||
Progression Question 4.1.3C
- Figure 4.3
- Figure 4.4
- Figure 4.6
- Figure 4.7
- Figure 4.10
Using “real world” language of science, this question reinforces the importance of the different bonds that stabilize the four levels of protein structure.
8.
The three-dimensional shape of a protein is determined by the primary, secondary, tertiary, and in many cases, the quaternary structure of the protein. The following are sentences taken from scientific articles on protein structure. For each of the statements, choose the level of protein structure that applies best.
"Disulfide bonds formed between cysteines stabilize the overall structure of this protein isolated from the bacterium."
| A. |
| B. |
| C. |
| D. |
| Question Notes | |||||||
Core Concept(s): 4.1 Level: HOC Type(s): MCQ, Small group discussion | Connections:
| Guidance: Using “real world” language of science, this question reinforces the importance of the different bonds that stabilize the four levels of protein structure. | Uses:
| ||||
Free response question: Q: The three dimensional shape of a protein is determined by the primary, secondary, tertiary, and in many cases, the quaternary structure of the protein. The sentences below are from scientific articles on protein structure. For each of the sentences, indicate the level of protein structure (primary, secondary, tertiary, or quaternary) that applies best.
(Correct answers are indicated in parentheses) | |||||||
Progression Question 4.1.3D
- Figure 4.3
- Figure 4.4
- Figure 4.6
- Figure 4.7
- Figure 4.10
Using “real world” language of science, this question reinforces the importance of the different bonds that stabilize the four levels of protein structure.
9.
The three-dimensional shape of a protein is determined by the primary, secondary, tertiary, and in many cases, the quaternary structure of the protein. The following are sentences taken from scientific articles on protein structure. For each of the statements, choose the level of protein structure that applies best.
"There are extensive ionic interactions between positively charged R-groups and negatively charged R-groups on the polypeptide."
| A. |
| B. |
| C. |
| D. |
| Question Notes | |||||||
Core Concept(s): 4.1 Level: HOC Type(s): MCQ, Small group discussion | Connections:
| Guidance: Using “real world” language of science, this question reinforces the importance of the different bonds that stabilize the four levels of protein structure. | Uses:
| ||||
Free response question: The three dimensional shape of a protein is determined by the primary, secondary, tertiary, and in many cases, the quaternary structure of the protein. The sentences below are from scientific articles on protein structure. For each of the sentences, indicate the level of protein structure (primary, secondary, tertiary, or quaternary) that applies best.
(Correct answers are indicated in parentheses) | |||||||
Progression Question 4.1.3E
- Figure 4.3
- Figure 4.4
- Figure 4.6
- Figure 4.7
- Figure 4.10
Using “real world” language of science, this question reinforces the importance of the different bonds that stabilize the four levels of protein structure.
10.
The three-dimensional shape of a protein is determined by the primary, secondary, tertiary, and in many cases, the quaternary structure of the protein. The following are sentences taken from scientific articles on protein structure. For each of the statements, choose the level of protein structure that applies best.
"There are hydrogen bonds between peptide backbone components that form neither an α-helix nor a β-pleated sheet."
| A. |
| B. |
| C. |
| D. |
| Question Notes | |||||||
Core Concept(s): 4.1 Level: HOC Type(s): MCQ, Small group discussion | Connections:
| Guidance: Using “real world” language of science, this question reinforces the importance of the different bonds that stabilize the four levels of protein structure. | Uses:
| ||||
Free response question: Q: The three dimensional shape of a protein is determined by the primary, secondary, tertiary, and in many cases, the quaternary structure of the protein. The sentences below are from scientific articles on protein structure. For each of the sentences, indicate the level of protein structure (primary, secondary, tertiary, or quaternary) that applies best.
(Correct answers are indicated in parentheses) | |||||||
Progression Question 4.1.3F
- Figure 4.3
- Figure 4.4
- Figure 4.6
- Figure 4.7
- Figure 4.10
Using “real world” language of science, this question reinforces the importance of the different bonds that stabilize the four levels of protein structure.
11.
The three-dimensional shape of a protein is determined by the primary, secondary, tertiary, and in many cases, the quaternary structure of the protein. The following are sentences taken from scientific articles on protein structure. For each of the statements, choose the level of protein structure that applies best.
"Peptide bonds form between the monomers."
| A. |
| B. |
| C. |
| D. |
| Question Notes | |||||||
Core Concept(s): 4.1 Level: HOC Type(s): MCQ, Small group discussion | Connections:
| Guidance: Using “real world” language of science, this question reinforces the importance of the different bonds that stabilize the four levels of protein structure. | Uses:
| ||||
Free response question: Q: The three dimensional shape of a protein is determined by the primary, secondary, tertiary, and in many cases, the quaternary structure of the protein. The sentences below are from scientific articles on protein structure. For each of the sentences, indicate the level of protein structure (primary, secondary, tertiary, or quaternary) that applies best.
(Correct answers are indicated in parentheses) | |||||||
Progression Question 4.1.4
- Figure 4.3
- Figure 4.4
This question reinforces the importance of the peptide bonds that stabilize the primary structure of proteins.
12.
The function of a protein is dependent upon the shape into which the chain of amino acids folds. Many noncovalent interactions are responsible for maintaining the protein's shape. Assume you have isolated a protein from an organism in its proper shape, and you have treated it with an enzyme that selectively breaks only the peptide bonds in the proteins. Would the protein retain its shape under these conditions?
| A. |
| B. |
| C. |
| D. |
| Question Notes | |||||||
Core Concept(s): 4.1 Level: HOC (asks students to make prediction based on what they understand about protein structure) Type(s): MCQ, Small group discussion | Connections:
| Guidance: This question reinforces the importance of the peptide bonds that stabilize the primary structure of proteins. | Uses:
| ||||
Free response question: Q: The function of a protein is dependent upon the shape into which the chain of amino acids folds. Many non-covalent interactions are responsible for maintaining the protein’s shape. Assume you have isolated a protein from an organism in its proper shape, and you have treated it with an enzyme that selectively breaks only the peptide bonds in the proteins. Would the protein retain its shape under these conditions? A: The shape, or conformation, of the protein would be destroyed because most of the amino acids would exist as free monomers. The peptide bonds responsible for maintaining the primary structure of a protein are absolutely essential to the correct structure, and therefore the function, of any protein. | |||||||
Progression Question 4.1.5
- Figure 4.2
- Figure 4.4
- Figure 2.11
This question provides a good review of pH (the concentration of positively charged hydrogen ions), temperature (the average energy of motion) and its effect on hydrogen bonds, and hydrophobic effect.
13.
The interactions between amino acids are major factors in determining the shape of a protein. These interactions can be affected by the environment surrounding a protein. Which of the following would have an effect on the shape of a protein?
| A. |
| B. |
| C. |
| D. |
| E. |
| Question Notes | |||||||
Core Concept(s): 4.1 Level: LOC, although a thorough explanation to the free response questions would be HOC (comprehension) for many first-year biology students. Type(s): MCQ, Small group discussion | Connections:
| Guidance: This question provides a good review of pH (the concentration of positively charged hydrogen ions), temperature (the average energy of motion) and its effect on hydrogen bonds, and hydrophobic effect. | Uses:
| ||||
Free response question: Q: The interactions between amino acids are major factors in determining the shape of a protein. These interactions can be affected by the environment surrounding a protein. Explain how the temperature, pH, ion concentration, and hydrophilic or hydrophobic properties of the environment may impact the shape of a protein, and therefore its function. A1: Temperature is an important environmental factor because of its effect on hydrogen bonds. Hydrogen bonds stabilize secondary structure and are often important in stabilizing tertiary and quaternary structure. At abnormally high temperatures, H bonds become destabilized, leading to changes in protein shape. A2: Increases or decreases in pH (the concentration of hydrogen ions) affect protein shape by interfering with normal electrostatic (ionic) interactions between charged or polar side chains. An increase in pH may cause deprotonation (and loss of partial charge) of a basic side chain. A decrease in pH (or an increase in hydrogen ion concentration) disrupts ion interactions between charged side chains due to the increased number of positive ions in the environment. A3: A change in ionic concentration (e.g., in sodium, or chloride ions) has a similar effect on the side chain interaction stabilized by electrostatic interactions or ionic bonds. A4: A hydrophilic environment will force nonpolar side chains to the interior of a protein. If the environment becomes more hydrophobic, the potential exists for the hydrophobic interactions between nonpolar sides chains to be disrupted, thereby altering the conformation of the protein. (NOTE: These “answers” to the free response question are written at a level much higher than would be expected from most first-year biology students. It will be up to the instructor to judge student understanding of this concept based on the instruction provided and the answers given by the students.) | |||||||
Progression 4.2
Learning Progression 4.2
Students should be able to answer the questions in this activity if they've mastered the following Core Concepts:
- 4.1 Proteins are linear polymers of amino acids that form three-dimensional structures with specific functions.
- 4.2 Translation is the process in which the sequence of bases in messenger RNA is used to specify the order of successive amino acids in a newly synthesized protein.
- 4.3 Proteins evolve by combining functional units and through mutation and selection.
Progression Question 4.2.1A
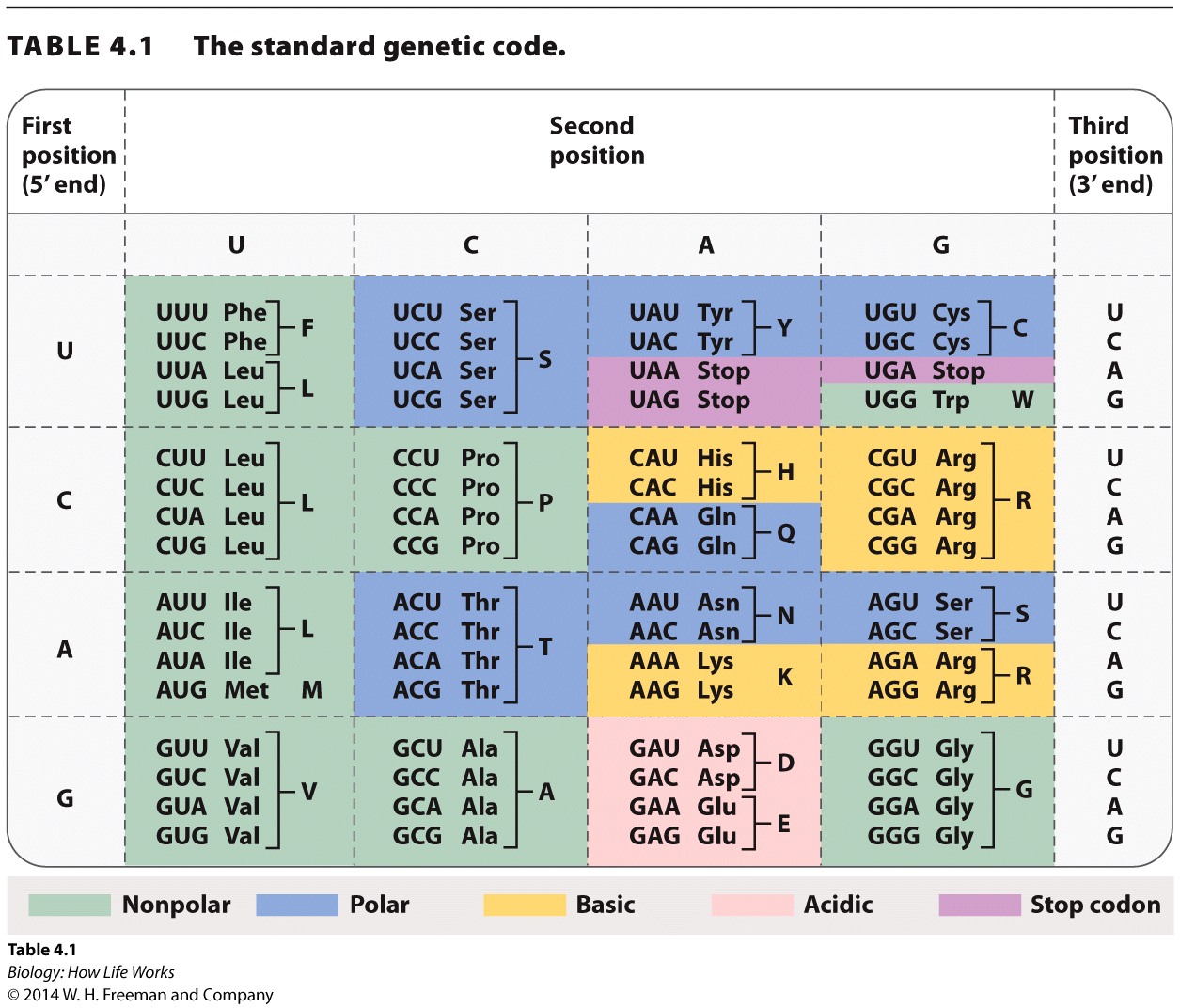
- Table 4.1
By navigating the genetic “dictionary” in Table 4.1, students will become familiar with the variation in the number of codons possible for individual amino acids.
14.
Some amino acids are coded by just one codon. Which of the following amino acids fall into this category? (More than one answer may be correct.)
methionine
tyrosine
tryptophan
serine
leucine
| Question Notes | |||||||
Core Concept(s): 4.1, 4.2 Level: LOC Type(s): MCQ, Small group discussion, More than one correct answer is intentional | Connections:
| Guidance: By navigating the genetic “dictionary” in Table 4.1, students will become familiar with the variation in the number of codons possible for individual amino acids. | Uses:
| ||||
Free response question: Q: Which of the amino acid(s) are encoded by just one codon? Which amino acids are encoded by the greatest number of codons? A: Methionine and tryptophan are each specified by only one codon. On the other hand, leucine, arginine, and serine are all encoded by six different codons. | |||||||
Progression Question 4.2.1B
- Table 4.1
By navigating the genetic “dictionary” in Table 4.1, students will become familiar with the variation in the number of codons possible for individual amino acids.
15.
Some amino acids are coded by as many as six different codons. Which of the following amino acids fall into this category? (More than one answer may be correct.)
methionine
tyrosine
tryptophan
serine
leucine
| Question Notes | |||||||
Core Concept(s): 4.1, 4.2 Level: LOC Type(s): MCQ, Small group discussion, More than one correct answer is intentional | Connections:
| Guidance: By navigating the genetic “dictionary” in Table 4.1, students will become familiar with the variation in the number of codons possible for individual amino acids. | Uses:
| ||||
Free response question: Q: Which of the amino acid(s) are encoded by just one codon? Which amino acids are encoded by the greatest number of codons? A: Methionine and tryptophan are each specified by only one codon. On the other hand, leucine, arginine, and serine are all encoded by six different codons. | |||||||
Progression Question 4.2.2A
- Figure 4.15
- Figure 4.17
- Table 4.1
Use this question to familiarize students with the genetic code and to illustrate that multiple codons that specify the same amino acid usually (but not always) vary at the third position.
16.
The codons in mRNA specify the amino acids that are used to make a protein. Mark the following statement concerning translation TRUE or FALSE.
"Sixty-one of the 64 possible codons specify amino acids, whereas the other three are stop codons. Each of the 61 codons specifies just one amino acid."
| A. |
| B. |
| Question Notes | |||||||
Core Concept(s): 4.1, 4.2 Level: LOC Type(s): MTFQ, Small group discussion | Connections:
| Guidance: Use this question to familiarize students with the genetic code and to illustrate that multiple codons that specify the same amino acid usually (but not always) vary at the third position. | Uses:
| ||||
Free response question: Q: Explain how 64 possible combinations of A, C, G, and U encode just 20 acids and three stop codons. A: Sixty-one of the 64 possible codons specify the 20 amino acids found in proteins. Two amino acids are specified by just one codon, nine amino acids are specified by two codons, one amino acid is specified by three codons, five amino acids are specified by four codons, and three amino acids are specified by six different codons. The remaining three codons are stop codons. | |||||||
Progression Question 4.2.2B
- Figure 4.15
- Figure 4.17
- Table 4.1
Use this question to familiarize students with the genetic code and to illustrate that multiple codons that specify the same amino acid usually (but not always) vary at the third position.
17.
The codons in mRNA specify the amino acids that are used to make a protein. Mark the following statement concerning translation TRUE or FALSE.
Some amino acids are specified by just one codon, whereas others are specified by multiple codons.
| A. |
| B. |
| Question Notes | |||||||
Core Concept(s): 4.1, 4.2 Level: LOC Type(s): MTFQ, Small group discussion | Connections:
| Guidance: Use this question to familiarize students with the genetic code and to illustrate that multiple codons that specify the same amino acid usually (but not always) vary at the third position. | Uses:
| ||||
Free response question: Q: Explain how 64 possible combinations of A, C, G, and U encode just 20 acids and three stop codons. A: Sixty-one of the 64 possible codons specify the 20 amino acids found in proteins. Two amino acids are specified by just one codon, nine amino acids are specified by two codons, one amino acid is specified by three codons, five amino acids are specified by four codons, and three amino acids are specified by six different codons. The remaining three codons are stop codons. | |||||||
Progression Question 4.2.2C
- Figure 4.15
- Figure 4.17
- Table 4.1
Use this question to familiarize students with the genetic code and to illustrate that multiple codons that specify the same amino acid usually (but not always) vary at the third position.
18.
The codons in mRNA specify the amino acids that are used to make a protein. Mark the following statement concerning translation TRUE or FALSE.
The limitations of the genetic code are such that a single amino acid may be specified by no more than four codons.
| A. |
| B. |
| Question Notes | |||||||
Core Concept(s): 4.1, 4.2 Level: LOC Type(s): MTFQ, Small group discussion | Connections:
| Guidance: Use this question to familiarize students with the genetic code and to illustrate that multiple codons that specify the same amino acid usually (but not always) vary at the third position. | Uses:
| ||||
Free response question: Q: Explain how 64 possible combinations of A, C, G, and U encode just 20 acids and three stop codons. A: Sixty-one of the 64 possible codons specify the 20 amino acids found in proteins. Two amino acids are specified by just one codon, nine amino acids are specified by two codons, one amino acid is specified by three codons, five amino acids are specified by four codons, and three amino acids are specified by six different codons. The remaining three codons are stop codons. | |||||||
Progression Question 4.2.2D
- Figure 4.15
- Figure 4.17
- Table 4.1
Use this question to familiarize students with the genetic code and to illustrate that multiple codons that specify the same amino acid usually (but not always) vary at the third position.
19.
The codons in mRNA specify the amino acids that are used to make a protein. Mark the following statement concerning translation TRUE or FALSE.
Because there are four RNA nucleotides and each codon is three nucleotides long, there are 64 possible codons, each of which directs the ribosome to incorporate a specific amino acid into a growing polypeptide chain.
| A. |
| B. |
| Question Notes | |||||||
Core Concept(s): 4.1, 4.2 Level: LOC Type(s): MTFQ, Small group discussion | Connections:
| Guidance: Use this question to familiarize students with the genetic code and to illustrate that multiple codons that specify the same amino acid usually (but not always) vary at the third position. | Uses:
| ||||
Free response question: Q: Explain how 64 possible combinations of A, C, G, and U encode just 20 acids and three stop codons. A: Sixty-one of the 64 possible codons specify the 20 amino acids found in proteins. Two amino acids are specified by just one codon, nine amino acids are specified by two codons, one amino acid is specified by three codons, five amino acids are specified by four codons, and three amino acids are specified by six different codons. The remaining three codons are stop codons. | |||||||
Progression Question 4.2.3A
- Figure 4.15
- Figure 4.26
- Table 4.1
This question will not be an easy one for most students, but will be very instructive. It incorporates basic numeracy skills with questions intended to foster conceptual understanding of the genetic code.
20.
Suppose that codons consisted of two nucleotides instead of three, but there were still 20 amino acids to be incorporated into proteins during translation. If this were the case, indicate whether the following statement would be TRUE or FALSE.
"There would still be enough codons to maintain the fidelity of the genetic code and provide stop codons."
| A. |
| B. |
| Question Notes | |||||||
Core Concept(s): 4.1, 4.2 Level: HOC Type(s): MTFQ, Small group discussion | Connections:
| Guidance: This question will not be an easy one for most students, but will be very instructive. It incorporates basic numeracy skills with questions intended to foster conceptual understanding of the genetic code. | Uses:
| ||||
Free response question: Q: Explain how the genetic code would be different if codons consisted of two nucleotides instead of three, but there were still 20 amino acids to be incorporated into proteins during translation? A: Since there would only be 16 codons (42, or four RNA nucleotides arranged in 16 different pairs), there wouldn’t be enough codons to maintain the fidelity of the genetic code and provide stop codons. That is, if there were only 16 codons, then some would have to code for more than one amino acid, resulting in an ambiguous coded instead of one with high fidelity. Redundancy would be eliminated, In fact, there would not be enough two-base codons to specify all 20 amino acids or act as stop codons. Most codons would probably specify a single amino acid, but some codons would have to specify more than one amino acid or an amino acid and a stop codon―not a good situation. | |||||||
Progression Question 4.2.3B
- Figure 4.15
- Figure 4.26
- Table 4.1
This question will not be an easy one for most students, but will be very instructive. It incorporates basic numeracy skills with questions intended to foster conceptual understanding of the genetic code.
21.
Suppose that codons consisted of two nucleotides instead of three, but there were still 20 amino acids to be incorporated into proteins during translation. If this were the case, indicate whether the following statement would be TRUE or FALSE.
Redundancy would be eliminated; in fact, there would not be enough two-base codons to specify all 20 amino acids or act as stop codons.
| A. |
| B. |
| Question Notes | |||||||
Core Concept(s): 4.1, 4.2 Level: HOC Type(s): MTFQ, Small group discussion | Connections:
| Guidance: This question will not be an easy one for most students, but will be very instructive. It incorporates basic numeracy skills with questions intended to foster conceptual understanding of the genetic code. | Uses:
| ||||
Free response question: Q: Explain how the genetic code would be different if codons consisted of two nucleotides instead of three, but there were still 20 amino acids to be incorporated into proteins during translation? A: Since there would only be 16 codons (42, or four RNA nucleotides arranged in 16 different pairs), there wouldn’t be enough codons to maintain the fidelity of the genetic code and provide stop codons. That is, if there were only 16 codons, then some would have to code for more than one amino acid, resulting in an ambiguous coded instead of one with high fidelity. Redundancy would be eliminated, In fact, there would not be enough two-base codons to specify all 20 amino acids or act as stop codons. Most codons would probably specify a single amino acid, but some codons would have to specify more than one amino acid or an amino acid and a stop codon―not a good situation. | |||||||
Progression Question 4.2.3C
- Figure 4.15
- Figure 4.26
- Table 4.1
This question will not be an easy one for most students, but will be very instructive. It incorporates basic numeracy skills with questions intended to foster conceptual understanding of the genetic code.
22.
Suppose that codons consisted of two nucleotides instead of three, but there were still 20 amino acids to be incorporated into proteins during translation. If this were the case, indicate whether the following statement would be TRUE or FALSE.
Most codons would probably specify a single amino acid. However, some codons would have to specify more than one amino acid or an amino acid and a stop codon.
| A. |
| B. |
| Question Notes | |||||||
Core Concept(s): 4.1, 4.2 Level: HOC Type(s): MTFQ, Small group discussion | Connections:
| Guidance: This question will not be an easy one for most students, but will be very instructive. It incorporates basic numeracy skills with questions intended to foster conceptual understanding of the genetic code. | Uses:
| ||||
Free response question: Q: Explain how the genetic code would be different if codons consisted of two nucleotides instead of three, but there were still 20 amino acids to be incorporated into proteins during translation? A: Since there would only be 16 codons (42, or four RNA nucleotides arranged in 16 different pairs), there wouldn’t be enough codons to maintain the fidelity of the genetic code and provide stop codons. That is, if there were only 16 codons, then some would have to code for more than one amino acid, resulting in an ambiguous coded instead of one with high fidelity. Redundancy would be eliminated, In fact, there would not be enough two-base codons to specify all 20 amino acids or act as stop codons. Most codons would probably specify a single amino acid, but some codons would have to specify more than one amino acid or an amino acid and a stop codon―not a good situation. | |||||||
Progression Question 4.2.4A
- Figure 4.3
- Figure 4.17
- Figure 4.19
- Figure 3.15
- Figure 3.18
- Table 4.1
Use this to provide practice (or test) identification of the template strand and the use of the genetic code in Table 4.1. Also use to reinforce concept of directionality of template strand (read 3’ to 5’ by the RNA polymerase) and the directionality of polypeptide synthesis (N-term first) by the ribosome.
23.
Use the double-stranded DNA molecule below to answer the following questions. The first pair of nucleotides (circled) is the start point of transcription.
Which of the following is the correct mRNA produced from the transcription of this DNA molecule?
| A. |
| B. |
| C. |
| D. |
| Question Notes | |||||||
Core Concept(s): 4.1, 4.2, 3.3 Level: LOC Type(s): MCQ, Small group discussion | Connections:
| Guidance: Use this to provide practice (or test) identification of the template strand and the use of the genetic code in Table 4.1. Also use to reinforce concept of directionality of template strand (read 3’ to 5’ by the RNA polymerase) and the directionality of polypeptide synthesis (N-term first) by the ribosome. | Uses:
| ||||
Free response question: Q: The first pair of nucleotides (circled) in the double-stranded DNA molecule above is the start point. Label the template and non-template strands of the DNA molecule. Write the corresponding mRNA molecule. Label the 5’ and 3’ ends of the transcript. Underline the start codon. Using the three-letter abbreviations for the amino acids in the table above, “assemble” (write out) the correct polypeptide encoded by the mRNA. Label the amino and carboxyl ends of the polypeptide. A: | |||||||
Progression Question 4.2.4B
- Figure 4.3
- Figure 4.17
- Figure 4.19
- Figure 3.15
- Figure 3.18
- Table 4.1
Use this to provide practice (or test) identification of the template strand and the use of the genetic code in Table 4.1. Also use to reinforce concept of directionality of template strand (read 3’ to 5’ by the RNA polymerase) and the directionality of polypeptide synthesis (N-term first) by the ribosome.
24.
Use the double-stranded DNA molecule below to answer the following questions. The first pair of nucleotides (circled) is the start point of transcription.
Which of the following is the correct polypeptide assembled by the translation of the mRNA produced by the transcription of this DNA molecule?
| A. |
| B. |
| C. |
| D. |
| Question Notes | |||||||
Core Concept(s): 4.1, 4.2, 3.3 Level: LOC Type(s): MCQ, Small group discussion | Connections:
| Guidance: Use this to provide practice (or test) identification of the template strand and the use of the genetic code in Table 4.1. Also use to reinforce concept of directionality of template strand (read 3’ to 5’ by the RNA polymerase) and the directionality of polypeptide synthesis (N-term first) by the ribosome. | Uses:
| ||||
Free response question: Q: The first pair of nucleotides (circled) in the double-stranded DNA molecule above is the start point. Label the template and non-template strands of the DNA molecule. Write the corresponding mRNA molecule. Label the 5’ and 3’ ends of the transcript. Underline the start codon. Using the three-letter abbreviations for the amino acids in the table above, “assemble” (write out) the correct polypeptide encoded by the mRNA. Label the amino and carboxyl ends of the polypeptide. A: | |||||||
Progression Question 4.2.5A
- Figure 4.2
- Figure 4.3
- Figure 4.17
- Figure 4.19
- Figure 3.15
- Figure 3.18
- Table 4.1
Provides practice transcribing and translating a sequence of DNA
Engages students with the genetic code
Allows students to discover how a small, single nucleotide substitution (mutation) can have a major effect on a gene product, or potentially no effect at all
Reinforces the concept of amino acid side chains as the parts of the molecule that give the amino acids their distinct chemical characteristics
As a follow-up to this set of questions, ask students to come up with their own single nucleotide mutations that would result in no change in amino acids, a conservative change, or a nonconservative change in the amino acid sequence.
25.
A mutation is defined as a change in the sequence of a DNA molecule. Sometimes mutations cause no obvious changes, but at other times mutations can have profound effects on the synthesis or the function of a protein.
The nucleotide sequences of the DNA molecules in the figure below are the same except for a single point mutation highlighted in yellow. Transcribe and translate each of the sequences then answer the questions below. (In each instance, the circled base pair is the start point of transcription.)

Which of the mutations above would be least likely to cause a change in the function of the protein?
| A. |
| B. |
| C. |
| D. |
| Question Notes | |||||||
Core Concept(s): 4.1, 4.2, 4.3 Level: HOC Type(s): MCQ, Small group discussion | Connections:
| Guidance: Provides practice transcribing and translating a sequence of DNA Engages students with the genetic code Reinforces the concept of amino acid side chains as the parts of the molecule that give the amino acids their distinct chemical characteristics As a follow-up to this set of questions, ask students to come up with their own single nucleotide mutations that would result in no change in amino acids, a conservative change, or a nonconservative change in the amino acid sequence. | Uses:
| ||||
Free response question: Q: A mutation is defined as a change in the sequence of a DNA molecule. Sometimes mutations cause no obvious changes, but at other times mutations can have profound effects on the synthesis or the function of a protein. The nucleotide sequences of the DNA molecules in the figure above are the same except for a single point mutation highlighted in yellow. Transcribe and translate each of the sequences then answer the questions below. (In each instance, the circled base pair is the start point of transcription.) 1. Which of the mutations would be least likely to cause a change in the function of the protein? 2. Which of the mutations would cause the synthesis of an incomplete (shorter than normal) version of the protein? 3. Which of the mutations would result in the replacement of an amino acid with a nonpolar side chain with a different, but similar, amino acid? A: | |||||||
Progression Question 4.2.5B
- Figure 4.2
- Figure 4.3
- Figure 4.17
- Figure 4.19
- Figure 3.15
- Figure 3.18
- Table 4.1
Provides practice transcribing and translating a sequence of DNA
Engages students with the genetic code
Allows students to discover how a small, single nucleotide substitution (mutation) can have a major effect on a gene product, or potentially no effect at all
Reinforces the concept of amino acid side chains as the parts of the molecule that give the amino acids their distinct chemical characteristics
As a follow-up to this set of questions, ask students to come up with their own single nucleotide mutations that would result in no change in amino acids, a conservative change, or a nonconservative change in the amino acid sequence.
26.
A mutation is defined as a change in the sequence of a DNA molecule. Sometimes mutations cause no obvious changes, but at other times mutations can have profound effects on the synthesis or the function of a protein.
The nucleotide sequences of the DNA molecules in the figure below are the same except for a single point mutation highlighted in yellow. Transcribe and translate each of the sequences then answer the questions below. (In each instance, the circled base pair is the start point of transcription.)

Which of the mutations above would cause the synthesis of an incomplete (shorter than normal) version of the protein?
| A. |
| B. |
| C. |
| D. |
| Question Notes | |||||||
Core Concept(s): 4.1, 4.2, 4.3 Level: HOC Type(s): MCQ, Small group discussion | Connections:
| Guidance: Provides practice transcribing and translating a sequence of DNA Engages students with the genetic code Reinforces the concept of amino acid side chains as the parts of the molecule that give the amino acids their distinct chemical characteristics As a follow-up to this set of questions, ask students to come up with their own single nucleotide mutations that would result in no change in amino acids, a conservative change, or a nonconservative change in the amino acid sequence. | Uses:
| ||||
Free response question: Q: A mutation is defined as a change in the sequence of a DNA molecule. Sometimes mutations cause no obvious changes, but at other times mutations can have profound effects on the synthesis or the function of a protein. The nucleotide sequences of the DNA molecules in the figure above are the same except for a single point mutation highlighted in yellow. Transcribe and translate each of the sequences then answer the questions below. (In each instance, the circled base pair is the start point of transcription.) 1. Which of the mutations would be least likely to cause a change in the function of the protein? 2. Which of the mutations would cause the synthesis of an incomplete (shorter than normal) version of the protein? 3. Which of the mutations would result in the replacement of an amino acid with a nonpolar side chain with a different, but similar, amino acid? A: | |||||||
Progression Question 4.2.5C
- Figure 4.2
- Figure 4.3
- Figure 4.17
- Figure 4.19
- Figure 3.15
- Figure 3.18
- Table 4.1
Provides practice transcribing and translating a sequence of DNA
Engages students with the genetic code
Allows students to discover how a small, single nucleotide substitution (mutation) can have a major effect on a gene product, or potentially no effect at all
Reinforces the concept of amino acid side chains as the parts of the molecule that give the amino acids their distinct chemical characteristics
As a follow-up to this set of questions, ask students to come up with their own single nucleotide mutations that would result in no change in amino acids, a conservative change, or a nonconservative change in the amino acid sequence.
27.
A mutation is defined as a change in the sequence of a DNA molecule. Sometimes mutations cause no obvious changes, but at other times mutations can have profound effects on the synthesis or the function of a protein.
The nucleotide sequences of the DNA molecules in the figure below are the same except for a single point mutation highlighted in yellow. Transcribe and translate each of the sequences then answer the questions below. (In each instance, the circled base pair is the start point of transcription.)

Which of the mutations above would result in the replacement of an amino acid with a nonpolar side chain by a different, but similar, amino acid?
| A. |
| B. |
| C. |
| D. |
| Question Notes | |||||||
Core Concept(s): 4.1, 4.2, 4.3 Level: HOC Type(s): MCQ, Small group discussion | Connections:
| Guidance: Provides practice transcribing and translating a sequence of DNA Engages students with the genetic code Reinforces the concept of amino acid side chains as the parts of the molecule that give the amino acids their distinct chemical characteristics As a follow-up to this set of questions, ask students to come up with their own single nucleotide mutations that would result in no change in amino acids, a conservative change, or a nonconservative change in the amino acid sequence. | Uses:
| ||||
Free response question: Q: A mutation is defined as a change in the sequence of a DNA molecule. Sometimes mutations cause no obvious changes, but at other times mutations can have profound effects on the synthesis or the function of a protein. The nucleotide sequences of the DNA molecules in the figure above are the same except for a single point mutation highlighted in yellow. Transcribe and translate each of the sequences then answer the questions below. (In each instance, the circled base pair is the start point of transcription.) 1. Which of the mutations would be least likely to cause a change in the function of the protein? 2. Which of the mutations would cause the synthesis of an incomplete (shorter than normal) version of the protein? 3. Which of the mutations would result in the replacement of an amino acid with a nonpolar side chain with a different, but similar, amino acid? A: | |||||||
BKQs
Basic Knowledge Questions
28. Basic Knowledge Question 4.1
Which one of the following is not a component of an amino acid?
| A. |
| B. |
| C. |
| D. |
| E. |
29. Basic Knowledge Question 4.2
True or False: amino acids with hydrophobic side chains are most often found buried in the interior of folded proteins.
| A. |
| B. |
30. Basic Knowledge Question 4.3
Which one of the following amino acids has its R-group covalently linked to the amino group of the amino acid?
| A. |
| B. |
| C. |
| D. |
| E. |
31. Basic Knowledge Question 4.4
Which one of the following amino acids is most likely to participate in hydrogen bonding with water?
| A. |
| B. |
| C. |
| D. |
| E. |
32. Basic Knowledge Question 4.5
The covalent bond between adjacent amino acids in a polypeptide chain is referred to as a bond.
33. Basic Knowledge Question 4.6
The three-dimensional structure of a functional protein composed of a single polypeptide chain is referred to as the ____________ structure.
| A. |
| B. |
| C. |
| D. |
34. Basic Knowledge Question 4.7
True or False: the individual polypeptide chains in a multi-subunit protein each have their own primary, secondary, and tertiary structure.
| A. |
| B. |
35. Basic Knowledge Question 4.8
Secondary structure is characterized by which one of the following types of interactions?
| A. |
| B. |
| C. |
| D. |
| E. |
36. Basic Knowledge Question 4.9
The two types of secondary structure interactions are referred to as:
| A. |
| B. |
| C. |
| D. |
| E. |
37. Basic Knowledge Question 4.10
X-ray crystallography was used to determine the structure of:
| A. |
| B. |
| C. |
| D. |
| E. |
38. Basic Knowledge Question 4.11
Alpha helices are stabilized by hydrogen bonds between amino acids that are ______ residues apart.
| A. |
| B. |
| C. |
| D. |
| E. |
39. Basic Knowledge Question 4.12
Which one of the following can contribute to a protein's tertiary structure?
| A. |
| B. |
| C. |
| D. |
| E. |
40. Basic Knowledge Question 4.13
In a ribbon model of a polypeptide, broad arrows indicate:
| A. |
| B. |
| C. |
| D. |
| E. |
41. Basic Knowledge Question 4.14
The unfolding of a protein by heat or chemical treatment is referred to as:
| A. |
| B. |
| C. |
| D. |
| E. |
42. Basic Knowledge Question 4.15
Proteins that do not refold properly after being heated generally require the assistance of which one of the following types of molecules in vivo?
| A. |
| B. |
| C. |
| D. |
| E. |
43. Basic Knowledge Question 4.16
Which one of the following would least likely be used during translation?
| A. |
| B. |
| C. |
| D. |
| E. |
44. Basic Knowledge Question 4.17
In the context of ribosomes, the Svedberg value 60S most likely represents:
| A. |
| B. |
| C. |
| D. |
| E. |
45. Basic Knowledge Question 4.18
True or False: because there are three different possible reading frames in a messenger RNA molecule, most mRNAs can be translated into three different proteins.
| A. |
| B. |
46. Basic Knowledge Question 4.19
Which one of the following is a critical region of a tRNA molecule?
| A. |
| B. |
| C. |
| D. |
| E. |
47. Basic Knowledge Question 4.20
How many different types of aminoacyl tRNA synthetases are there?
| A. |
| B. |
| C. |
| D. |
| E. |
48. Basic Knowledge Question 4.21
The codon used to initiate protein synthesis is: .
49. Basic Knowledge Question 4.22
Which one of the following codons is capable of terminating translation?
| A. |
| B. |
| C. |
| D. |
| E. |
50. Basic Knowledge Question 4.23
The genetic code is:
| A. |
| B. |
| C. |
| D. |
| E. |
51. Basic Knowledge Question 4.24
Which one of the following would least likely be used during the initiation phase of translation?
| A. |
| B. |
| C. |
| D. |
| E. |
52. Basic Knowledge Question 4.25
When the peptide bond is created between amino acid 1 and amino acid 2:
| A. |
| B. |
| C. |
| D. |
53. Basic Knowledge Question 4.26
True or False: peptide bond formation is catalyzed by the enzymatic activity of an RNA molecule.
| A. |
| B. |
54. Basic Knowledge Question 4.27
A group of functionally related genes transcribed as a single transcriptional unit under the control of a single promoter is referred to as a(n) .
55. Basic Knowledge Question 4.28
An initiator codon in E. coli is identified by locating:
| A. |
| B. |
| C. |
| D. |
| E. |
56. Basic Knowledge Question 4.29
A polycistronic mRNA with six protein coding genes would have:
| A. |
| B. |
| C. |
| D. |
| E. |
57. Basic Knowledge Question 4.30
A region of a protein that folds in a similar way independently of the rest of the protein is referred to as a folding .
58. Basic Knowledge Question 4.31
Structurally and functionally related proteins are grouped into approximately 25,000 different protein
59. Basic Knowledge Question 4.32
Amino acid sequences evolve through:
| A. |
| B. |
| C. |
| D. |
| E. |
60. Basic Knowledge Question 4.33
In which of the following ribosomal sites is there complete codon-anticodon base pairing?
| A. |
| B. |
| C. |
| D. |
| E. |
61. Basic Knowledge Question 4.34
Which one of the following steps in translation is not part of the elongation phase?
| A. |
| B. |
| C. |
| D. |
| E. |
Pre-Made Assessments
Pre-Made Assessments

This section will show how we've used the Progression Questions and Basic Knowledge Questions in pre-made assessments in the Launch Pad Unit.
Chapter 4 Pre-Class Assignment
This assignment pulls appropriate questions from the Biology: How Life Works Progressions and Basic Knowledge Questions to provide you with a pre-made assignment meant to be used after students read a chapter and before class. Designed by an author, this assignment considers the goals of the chapter and addresses Core Concepts. Included questions are listed below.
- Basic Knowledge Questions 4.1, 4.6, 4.13, 4.14, 4.20, 4.21, 4.27, 4.30, 4.33, 4.34
Chapter 4 In-Class Learning Progression Questions
The Progressions allow you to use assessment not only for testing, but for teaching. This assignment was developed by an author and suggests questions from the Progressions that can be used during your class to develop meaningful classroom discussions. Included questions are listed below.
- Progression 4.1.1(A-D), 4.1.2, 4.1.5
- Basic Knowledge Questions 4.2, 4.7, 4.12, 4.18, 4.26, 4.29
Chapter 4 Homework
This assignment pulls appropriate questions from the Biology: How Life Works Progressions and Basic Knowledge Questions to provide you with a pre-made assignment meant to be used after class as homework. Designed by an author, this assignment considers the goals of the chapter and addresses Core Concepts. Included questions are listed below.
- Progression 4.1.3(A-F)
- Progression 4.2.1(A-B), 4.2.4(A-B)
- Basic Knowledge Questions 4.3, 4.4, 4.10, 4.11, 4.15, 4.16, 4.17, 4.19, 4.22, 4.24, 4.28, 4.31, 4.32, 4.35, 4.36, 4.37, 4.38, 4.39
Chapter 4 Exam Questions
Developed by one of our authors, this pre-made exam shows you an example of how you can use the Progressions and Basic Knowledge Questions to develop an exam that will measure student understanding of chapter Core Concepts. Instructors who write their own exams, may wish to use these questions as a quiz or practice test. Included questions are listed below.
- Progression 4.1.1(Free Response), 4.1.4
- Progression 4.2.2(A-D), 4.2.3(A-C), 4.2.5(A-C, Free Response)
- Basic Knowledge Questions 4.5, 4.8, 4.9, 4.23, 4.25