Chapter 2. Analyzing Enzymes and Enzyme Kinetics II
Learning Objectives
General Purpose
Conceptual
- Be able to relate changes in different factors to changes in the rate of enzyme-catalyzed reactions.
- Be able to design and conduct experiments to test your hypotheses.
General Purpose
The pre-lab is to prepare for the upcoming experiment procedures you will do during this laboratory. At the end of the last laboratory your instructor described in general terms the various treatments groups will use in this laboratory. Your group selected one of those treatments and you now need to plan the details of your experiment. Before coming to lab you should write out the complete procedure that you are going to use in your laboratory notebook. Your group should be prepared to review your procedure with your laboratory instructor. If you have questions or are uncertain about your procedure please check it with your laboratory instructor prior to beginning your experiment. This will save you the time of having to repeat the experiment.
You should be as specific as possible about the details of your procedure (volumes, temperatures, pH, etc.). Unless instructed differently by your instructor, all of the reactions will run for the same length of time (three minutes). The general outlines for the various treatments will be available on each bench during your laboratory. Read each of the procedures to become familiar with the scope of the various experiments and then use the one that is specific for the treatment your group chose as a guide to write your specific procedure.
Procedure Outlines
- Enzyme Concentration
- For example, if using the potato enzyme at a concentration of 1×, the experimental tubes will contain 3 mL of water, 5 mL of 0.05 M catechol, and 1 mL of enzyme. Note that the total volume is 9 mL. The blank will contain 8 mL of water and 1 mL of potato enzyme.
- If you are investigating the rate of the reaction when the enzyme is diluted by one half, each experimental tube will contain 3.5 mL of water, 5 mL of 0.05 M catechol, and 0.5 mL of enzyme. Note that the total volume is 9 mL. The blank will contain 8.5 mL of water and 0.5 mL of potato enzyme.
b. Prepare appropriate blanks using the diluted enzyme solution. You will need to prepare a “blank solution” for each dilution, so don’t forget to include the appropriate volumes of water or enzyme in the blank.
c. Add the appropriate amount of potato enzyme extract to the tube to start the reaction.
- Substrate Concentration
- For example, if using 0.05 M catechol, the experimental tubes in this series will contain 3 mL of water, 5 mL of 0.05 M catechol, and 1 mL of enzyme. Note that the total volume is 9 mL.
- If you are investigating the rate of the reaction when the substrate is diluted by one half, each experimental tube will contain 5.5 mL of water, 2.5 mL of 0.05 M catechol, and 1.0 mL of enzyme. Note that the total volume is 9 mL.
b. Prepare appropriate blank.
c. Add 1 mL of potato enzyme extract to the tube to start the reaction.
- Structure of the Substrate
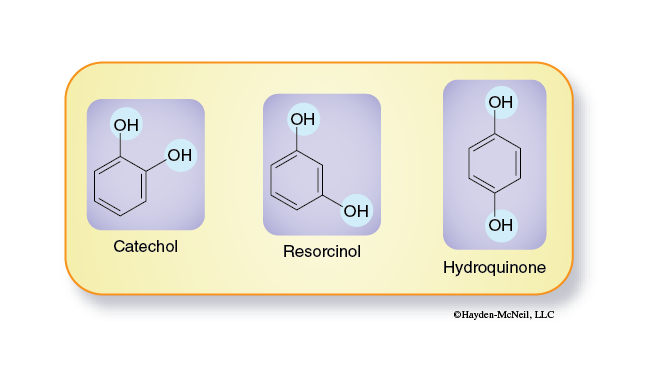
There are several structural isomers of catechol (Figure 3-2). These molecules differ only in the relative positions of the hydroxyl groups.
b. Prepare appropriate blank.
c. Add 1 mL of potato enzyme extract to the tube to start the reaction.
- pH
a. Choose a series of three different pH buffers from the following list. Buffers of the following pHs will be available: 3, 5, 7, 9, 11. The buffers will substitute for the water in the basic reaction..
b. Prepare a series of culture tubes containing 3 mL of each different buffer and 5 mL of 0.05 M catechol.
c. Prepare appropriate blank. You will need to prepare a “blank solution” for each different pH.
d. Add 1 mL of potato enzyme extract to the tube to start the reaction.
- Temperature
a. Prepare a series of culture tubes containing 3 mL water and 5 mL of 0.05 M catechol.
b. Prepare appropriate blank. You will need to prepare a “blank solution” for each different temperature.
c. Place these tubes in environments with different temperatures (Table 3-1). Allow the tubes to equilibrate for 5 minutes. Also allow enough of the potato extract to equilibrate at the different temperatures for each of the reactions.
d. Add 1 mL of potato enzyme extract to the tube to start the reaction.
Table 3-1. Possible environmental temperatures.
- Source of Enzyme
a. Prepare the enzyme extract by placing 20 grams of the source material (minus the peel and any seeds, etc.) in a blender with 125 mL of cold deionized water.
b. Blend until all the source material has been ground into fine particles (approximately two minutes).
c. Filter the liquid through two layers of cheesecloth into a flask making sure that all of the particulate matter has been removed from the liquid.
d. This liquid is your new enzyme extract and should be used in place of the potato enzyme extract used in the last laboratory experiment.
e. Prepare appropriate blank. You will need to prepare a “blank solution” for each different source.
f. Add 1 mL of enzyme extract to the tube to start the reaction.
A useful tool for the preparation and execution of your specific procedure is a diagram where the volumes and the order of each ingredient in a reaction can be listed. Then as the ingredients are added they can be checked off the list. At the end of this section there is a tube diagram you can use to help with your procedure.
Once you have written out your procedure in your laboratory notebook make a data table similar to the one shown below (Table 3-2) for recording your data. If you have more treatments your table will need to be larger and you should consider changing the table orientation in your lab notebook to accommodate the larger table.
Table 3-2. Absorbance measurements.
You should also measure the absorbance of your blank at the end of your replicates to note any absorbance changes which may have occurred. Make a note of this end absorbance for the blank in your lab notebook.
Pre-Lab Quiz
Proceed to the Pre-Lab Quiz