Chapter 1. Development of an Equation
Discussion
Objectives

- Apply laboratory procedures and make observations to investigate a chemical reaction. Based on these observations, identify the pattern of reactivity and communicate what occurred in the reaction by writing a chemical equation and a particle diagram consistent with the data.
Introduction
Suppose two substances are added together and they begin burning, with smoke rising and flames jumping. To a chemist this is just another day in the laboratory (and a great demonstration!), and she may describe this phenomenon in different ways. One way is to record macroscopic observations. What took place? What was measured? What was seen, heard, smelled? Or perhaps the chemist thinks about her observations and infers (concludes based on evidence and reasoning) what happened to the particles. What did the atoms, ions, or molecules do as they interacted with each other, and how did these interactions produce a fire? A chemist cannot actually see the atoms, but based on the experimental evidence it might be possible to provide an explanation, a theory, that is consistent with the observations. Finally, the chemist may decide to write a chemical equation to symbolize what took place during the reaction. All of these are legitimate ways to describe what transpired and in this experiment, you will utilize each one.
Chemical equations are usually presented as “facts” in textbooks, but how does a chemist know that the reaction really took place as written? Where is the evidence? What led to the inference? In this experiment, you will investigate a particular reaction, make observations, infer what you think happened to the atoms and ions, and finally propose a chemical equation consistent with your data.
Chemical Equations and Particle Diagrams
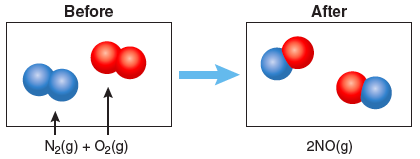
Chemical equations are used to describe a chemical reaction with symbols. For example, nitrogen, N2, and oxygen, O2, can combine to form nitric oxide, NO. The chemical equation for this is:
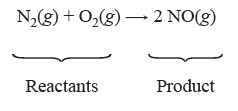
In this equation, the (+) symbol indicates that nitrogen reacts with oxygen and the arrow indicates that nitric oxide is formed. The chemical formulas on the left side of the equation are collectively known as the reactants and those on the right side as the products. In this case we have one kind of product, NO(g). The letter “g” in parentheses is included to indicate that these are in the gaseous phase. Other phase symbols include (s) for solid, (l) for liquid, and (aq) for aqueous or in water. The subscripts in the formula tell us how many of each atom there are in a molecule. In the formula above, there are two nitrogen atoms in the gas molecule N2, two oxygen atoms in the gas molecule O2, and one nitrogen atom with one oxygen atom in the gas molecule NO.
The number in front of the chemical formula, or coefficients, indicate how many molecules are present. In a chemical reaction atoms are not created or destroyed, thus there should be an equal number of atoms of each element on the left and right sides of the equation. An equation is balanced when there are an equal number of atoms of each element on the left and right sides of the equation. In the equation above, we can see that one nitrogen gas molecule and one oxygen gas molecule combine to form two nitric oxide gas molecules. There are a total of two nitrogen atoms on the left side of the equation and two nitrogen atoms on the right side of the equation. The same is true for the oxygen atoms, so this chemical equation is balanced. This same reaction may also be described by indicating what happens to the particles:
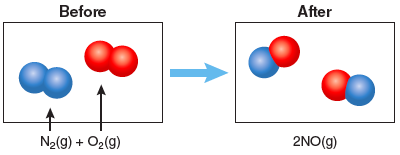
Question 3.1: Nitrogen and oxygen can combine to form other molecules containing N and O. One is nitrous oxide, or N2O. N2O is commonly used in dentistry for its anesthetic and analgesic effects and is better known as laughing gas. Write a balanced chemical equation for N2 reacting with O2 to form laughing gas. In the boxes below, draw a particle diagram showing the reactants (before) and the products (after) the reaction. The drawing should be consistent with your balanced equation.
Common Patterns of Chemical Reactivity
Different types of common chemical reactions are discussed in your textbook. These include:
- Combination reactions, which describe the reaction of two or more reactants to form one product.
- Decomposition reactions, in which one substance reacts to form two or more compounds or elements. This type of reaction typically occurs when compounds are heated.
- Combustion reactions, which often occur quickly and evolve heat in the form of a flame. Many of these reactions involve hydrocarbons and oxgyen reacting to form carbon dioxide and water.
- Exchange (metathesis) reactions in which the positive and negative ions appear to exchange partners.
Question 3.2: Is the chemical reaction N2(g) + O2(g) → 2 NO(g) an example of a combination, decomposition, combustion, or exchange reaction?
Overview of Experiment
In this experiment you will investigate the reaction that takes place when an aqueous lead nitrate solution is combined with an aqueous potassium iodide solution. Your objective is to determine the appropriate pattern of reactivity and propose a chemical equation and particle diagram that is supported by your laboratory observations.
To get started you must make observations. In Part A you will combine a lead nitrate solution with a potassium iodide solution and record your observations. What atoms, ions, or molecules do you think are in each solution before they are combined? After they are combined, is there evidence for a chemical reaction? Is there any indication that a gas is formed?
What happens to the solutions of lead nitrate and potassium iodide when they are combined? Is there a chemical reaction? To answer these questions you must be able to identify the presence of different ions. In Part B you will perform qualitative tests for the different possible ions in this experiment. Procedures are given for these qualitative tests. Make careful observations when the known ions are tested, since you will be repeating these tests with your actual sample and comparing the results.
In Part C you will apply your understanding of qualitative tests to determine which ions are in the solid product that forms when lead nitrate and potassium iodide solutions are mixed.
Determining the identity of ions comprising your solid product is important. After Part C you probably have enough evidence to select the appropriate pattern of reactivity. However, you need more information to write a chemical equation. The qualitative analysis determined what ions are present, but it did not provide any quantitative information. A chemical equation includes quantitative information and communicates the amounts of the different substances. To get this information you must complete a quantitative test.
In Part D you will perform a quantitative test to determine the exact ratio of ions in the precipitate that formed during your reaction. This will be accomplished by mixing solutions of lead nitrate and potassium iodide (the reactants) in varying ratios and making observations. There is an exact stoichiometric ratio in which these two substances will combine with each other such that neither one remains. However, for every other ratio the reaction will take place, but one of the reactants will be “leftover.” The reactant that remains after the reaction was “in excess.” The reactant that was completely consumed is the limiting reactant.
To illustrate the idea of reaction stoichiometry, consider the burning of hydrogen gas in oxygen to form water vapor:
2 H2(g) + O2(g) → 2 H2O(g)
In the balanced equation, the H2:O2 ratio is 2:1. When hydrogen gas is combined with oxygen in a 2:1 ratio, all of the H2 will react with all of the O2 to produce H2O(g) and there are no “leftover” reactants.
What if different ratios of H2 to O2 are used? There will be leftover reactants:
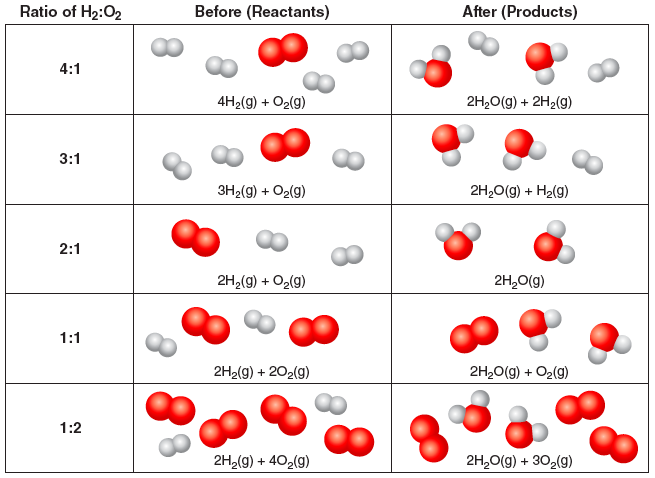
Question 3.3: In the table above, circle the leftover reactants. What is the limiting reactant for each ratio H2:O2?
| H2:O2 Ratio | Limiting Reactant |
|---|---|
| 4:1 | |
| 3:1 | |
| 2:1 | |
| 1:1 | |
| 1:2 |
How could a chemist determine the ratio of H2:O2 that does not have leftover reactants? They need a test to determine whether H2(g) or O2(g) remains. This is actually a simple experiment; if a wooden splint is lit, extinguished, and then placed in a container of hydrogen gas while still glowing, it produces a loud “pop.” However, if the glowing splint is placed in a container of oxygen gas, it reignites.
Question 3.4: Suppose H2(g) and O2(g) are combined in the ratios shown in the previous table to produce H2O. For which ratios of H2:O2 would the splint test result in a “pop.” For which ratios of H2:O2 would the splint reignite? How does this analysis lead the chemist to the correct chemical equation?
In Part D you will apply quantitative reasoning to determine the ratio of potassium iodide reacting with lead nitrate in your chemical equation. This will be accomplished by mixing drops of each solution in different ratios and then testing the supernatant (the liquid above the precipitate) for excess ions. Instead of doing a test with a glowing splint, however, you will determine whether there are excess ions by checking whether a precipitate can be formed with the ions (that may) be leftover. The solution in which neither ion is in excess indicates that the ions were added in the correct ratio to exactly form the product. Note that having neither ion in excess requires careful measurement of amounts of the two solutions. A very small amount of precipitate will form while testing the supernatant liquid if there is a slight excess of either reactant. It is therefore important not only to measure carefully, but also to note the amount of precipitate when testing for the ion in excess. Can you apply quantitative reasoning from Part D to determine the chemical equation? Try question 5.
Question 3.5: When solutions of barium chloride and sodium sulfate are mixed, a white precipitate of barium sulfate forms:
Barium chloride + sodium sulfate → barium sulfate (a white precipitate) + sodium chloride
To determine the chemical equation the following five test tubes were prepared and tested:
| Drops of Barium Chloride |
Drops of Sodium Sulfate |
Drops of Water |
Observation | Inference for the Barium Chloride to Sodium Sulfate Ratio |
|
|---|---|---|---|---|---|
| Tube 1 | 6 | 2 | 22 | White precipitate formed |
A 3:1 ratio produces barium sulfate |
| Tube 2 | 6 | 3 | 21 | White precipitate formed |
A 2:1 ratio produces barium sulfate |
| Tube 3 | 6 | 6 | 18 | White precipitate formed |
A 1:1 ratio produces barium sulfate |
| Tube 4 | 6 | 12 | 12 | White precipitate formed |
A 1:2 ratio produces barium sulfate |
| Tube 5 | 6 | 18 | 6 | White precipitate formed |
A 1:3 ratio produces barium sulfate |
These results are inconclusive and do not narrow down the barium chloride to sodium sulfate ratio in the balanced chemical equation because every reaction produces barium sulfate. However, by testing each supernatant (the liquid above each precipitate) it is possible to determine which ions are “leftover” and in excess. In the table below, identify the ions present in the supernatant (Ba2+, Cl−, Na+, and/or SO42−) prior to additional barium chloride or sodium sulfate.
| Barium Chloride Added to Supernatant Observation | Sodium Sulfate Added to Supernatant Observation | Ions Present in the Supernatant prior to Adding Additional Reagents | |
|---|---|---|---|
| Tube 1 | No additional precipitate formed | White precipitate formed | |
| Tube 2 | No additional precipitate formed | White precipitate formed | |
| Tube 3 | No additional precipitate formed | No additional precipitate formed | |
| Tube 4 | White precipitate formed | No additional precipitate formed | |
| Tube 5 | White precipitate formed | No additional precipitate formed |
Question 3.6: Use the information in the table above to determine the empirical formula of barium sulfate. Support your reasoning.
Question 3.7: Write a balanced chemical equation for the reaction that occurs when barium chloride and sodium sulfate are mixed.
Materials Provided
Equipment
- 15 4-mL test tubes
- medicine dropper
- micropipet
- micro spatula
- water bath (100-mL beaker)
- test tube rack
- Bunsen burner, wire gauze
- thin-walled rubber tubing
- ring stand, ring
Common Equipment
- centrifuge
Chemicals
- 0.1 M lead nitrate, Pb(NO3)2
- 0.1 M potassium nitrate, KNO3
- 0.1 M potassium iodide, KI
- 3 M nitric acid, HNO3
- 5% thioacetamide, CH3CSNH2
- 3% hydrogen peroxide, H2O2
- 1,2-dichloroethane, C2H4Cl2
Cautions
Be careful to avoid burns from ring and open flame. Since lead salts are toxic substances, be sure to wash your hands thoroughly. 1,2-dichloroethane (dichloroethane) is a suspect carcinogen and may be irritating to the respiratory tract. Heating thioacetamide solutions produces H2S, an inhalation poison. Do this procedure well within the hood. Fume hoods must be on.
Procedure
Label a clean, dry 4-mL test tube "lead nitrate" and fill it with the appropriate solution. Label a second clean, dry 4-mL test tube "potassium iodide" and fill it with the appropriate solution. Always take drops from these tubes. Refill as necessary.
A. Initial observations.
In this section of the experiment you will observe the reaction that occurs when solutions of potassium iodide and lead nitrate are mixed.
- Add 5 drops of each solution to a 4-mL test tube and observe what happens. It may be helpful to centrifuge the test tube after the reaction. Record your observations in your notebook.
B. Qualitative tests for ions.
In Part C we will use chemical tests to determine which ions are present in the product of the above reaction. However, before testing our product we must first see how each of the anions and cations present in this experiment react. That is the task for this section.
Procedure for Testing Anions
In this section of the experiment, you will see what the reactions of the anions (iodide and nitrate) look like when they react with hydrogen peroxide. Be sure to make careful observations in your notebook. Later, you will use this test to determine which anion is present in a solution by repeating the experiment and comparing your observations.
- Add 5 drops of KI, 5 drops nitric acid, 10 drops dichloroethane, and 5 drops hydrogen peroxide to a clean 4-mL test tube. Stopper and shake the test tube. You may decide to centrifuge the solution in the test tube to produce two distinct layers.
- Add 5 drops of KNO3, 5 drops nitric acid, 10 drops dichloroethane, and 5 drops hydrogen peroxide to a clean 4-mL test tube. Stopper and shake the test tube. You may decide to centrifuge the solution in the test tube to produce two distinct layers.
Procedure for Testing Cations
In this section of the experiment you will see what the reactions of the cations (lead and potassium) look like when they react with H2S. Be sure to make careful observations in your notebook. Later, you will use this test to determine which cation is present in a solution by repeating the experiment and comparing your observations.
- To each of two clean, dry 4-mL test tubes, add 20 drops of thioacetamide and 2 drops
of nitric acid. On heating, the thioacetamide will decompose to form H2S. - To one tube add 5 drops Pb(NO3)2 , and to the other 5 drops KNO3. Heat both tubes in a water bath for at least 5 minutes and record your observations.
C. Identification of ions in the precipitate.
In this section of the experiment you will test the product that formed when potassium iodide and lead nitrate solutions were mixed. To accomplish this you will re-dissolve the solid and determine which ions it is comprised of.
Formation of Precipitate
- Once again, add 5 drops of potassium iodide and 5 drops lead nitrate to a 4-mL test tube. (You may simply use the sample from Part A if you still have it.) Centrifuge the test tube, and then carefully remove and discard the clear aqueous layer. Wash the precipitate: add 1 mL of distilled water, stir, centrifuge the test tube, and discard the clear aqueous layer. Repeat the washing.
- Add 5 drops of nitric acid, 10 drops of dichloroethane, and 5 drops of hydrogen peroxide to the solid in the test tube. Stopper and shake the test tube.
- If necessary, alternate holding the test tube in a stream of hot tap water for 20 seconds and shaking for 40 seconds until the yellow solid is completely dissolved. Centrifuge the test tube. Record your observation of the dichloroethane layer and compare it with your findings from Part B.
- Transfer the less-colored aqueous layer to a clean 4-mL test tube using a micropipet. Discard the remaining dichloroethane layer in a waste beaker.
- Heat the aqueous solution in a water bath to destroy any unreacted hydrogen peroxide. Continue heating until the solution is completely colorless.
- Add 20 drops of thioacetamide to the test tube, stir, and heat in a water bath. While waiting for a reaction to occur, start on the next step. After 5 minutes, compare your results with the reaction observed in Part B.
D. Determination of the ratio of ions in the precipitate.
In Part C, you determined which ions are in the precipitate. In this section you will make observations and determine the ratio of these ions in the solid sample.
Preparing Test Tubes with Varying Ratios of The Reactants
- Label five 4-mL test tubes 1 through 5. The five test tubes should be filled carefully according to the table below. Notice the table has several empty cells. Reproduce the table in your notebook and fill in the empty cells before continuing. In each case make the amount of lead nitrate constant and the total number of drops constant.
Table 3.1 Solutions to be mixed in Part D.
| Drops of Lead Nitrate |
Drops of Potassium Iodide |
Drops of Water |
Lead Nitrate: Potassium Iodide Ratio |
|
|---|---|---|---|---|
| Tube 1 | 12 | 4 | 32 | 3:1 |
| Tube 2 | 12 | 6 | 2:1 | |
| Tube 3 | 12 | 12 | 24 | |
| Tube 4 | 12 | 1:2 | ||
| Tube 5 | 36 | 0 | ||
- The same medicine dropper should be used to measure all drops. (Do not use a micropipet—the drop sizes vary too greatly.) Measure out all five amounts of each solution, and then rinse the medicine dropper well with distilled water before changing solutions.
- Stopper and shake the five test tubes. Allow the precipitate to settle for a few minutes, and then centrifuge the test tubes. The centrifuge must be balanced using pairs of test tubes—try to centrifuge as many tubes as possible at the same time.
- Record the appearance and relative amounts of precipitate in each of the five test tubes in your notebook.
Determining Which Test Tube, 1–5, Does Not Have an Excess of Cation or Anion.
When the ions in the potassium iodide and lead nitrate solutions were mixed they may have produced different amounts precipitate, as noted in step 4. For a given ratio, were any of the ions in excess? If so, they will still be in the supernatant. This can be tested for by taking a portion of the supernatant and seeing if it is still possible to form additional precipitate. Obviously, if more precipitate is formed, excess ions must have been present in supernatant (step 2).
- To test a given supernatant for excess ions, use a medicine dropper and remove 5 drops of the solution into a different test tube. Be careful not to transfer any of the precipitate. You may need to re-centrifuge the tube to avoid transferring any solid.
- If you are testing for the presence of potassium or iodide ions in the supernatant, add 5 drops of lead nitrate and record the relative amount of precipitate that forms (if any).
- If you are testing for the presences of lead or nitrate ions in the supernatant, add 5 drops of potassium iodide.
Once again, make careful observations. You may find it helpful to centrifuge the samples and make comparisons.
Waste Disposal
All solutions containing lead ions and dichloroethane must be collected in a beaker at your desk. Neutralize the solution with sodium hydroxide, add it to the inorganic waste beaker in the hood, and fill in the waste disposal sheet. Your lab instructor will dispose of the total volume in the appropriate container. If solids remain in the test tubes, dispose of them in the uncleanable glass waste container.
Answer Clinic
Question 3.1
Write a balanced chemical equation for N2 reacting with O2 to form laughing gas.
Answer
2 N2(g) + O2(g) → 2 N2O (g)
In the boxes below, draw a particle diagram showing the reactants (before) and the products (after) the reaction. The drawing should be consistent with your balanced equation.
Question 3.2
Is the chemical reaction N2(g) + O2(g) → 2 NO(g) an example of a combination, decomposition, combustion, or exchange reaction?
Answer
Combination reactions, which describe the reaction of two or more reactants to form one product.
Question 3.3
What is the limiting reactant for each ratio H2:O2?
Answer
| H2:O2 Ratio | Limiting Reactant |
| 4:1 | O2(g) |
| 3:1 | O2(g) |
| 2:1 | n/a |
| 1:1 | H2(g) |
| 1:2 | H2(g) |
Question 3.4
Suppose H2(g) and O2(g) are combined in the ratios shown in the table on "Overview" of Experiment section to produce H2O. For which ratios of H2:O2 would the splint test result in a “pop.” For which ratios of H2:O2 would the splint reignite? How does this analysis lead the chemist to the correct chemical equation?
Answer
When the H2:O2 = 2 there will not be any H2 or O2 left over. If H2:O2 > 2 there is excess H2 and the O2 is the limiting reactant, and the splint tests produce a “pop.”
If H2:O2 < 2 there is excess O2 and the H2 is the limiting reactant, and the splints will be reignited.
Question 3.5
Identify the ions present in the supernatant (Ba2+, Cl−, Na+ and/or SO42−) prior to additional barium chloride or sodium sulfate.
Answer
Tube 1: Ba2+ , Na+, Cl–
Tube 2: Ba2+ , Na+, Cl–
Tube 3: Na+, Cl–
Tube 4: Na+, Cl–, SO42–
Tube 5: Na+, Cl–, SO42–
Question 3.6:
Use the information in the table on "Overview" of Experiment section to determine the empirical formula of barium sulfate. Support your reasoning.
Answer
By testing the supernatant it appears that tube 3 does not have excess barium ions or excess sulfate ions. This tube was prepared using equal drops of barium chloride and sodium sulfate. This suggests that the ratio of barium to sulfate is 1:1, consistent with an empirical formula of BaSO4.
Question 3.7:
Write a balanced chemical equation for the reaction that occurs when barium chloride and sodium sulfate are mixed.
Answer
BaCl2(aq) + Na2SO4(aq) → BaSO4(s) + 2 NaCl(aq)