Chapter 1. Gravimetric Determination of Sulfate
Discussion
Objectives
- Use quantitative precipitation to isolate and quantify the sulfate from a sample.
- Learn about precipitating reactions and be able to use the general solubility guidelines to predict the formation of solid products in ionic reactions.
Introduction
In the previous lab you mixed solutions containing two ionic compounds, KI and Pb(NO3)2, together to form a brilliant yellow PbI2 solid.
2 KI(aq) + Pb(NO3)2 (aq) → PbI2(s) + 2 KNO3(aq)
The PbI2 formed is the precipitate, or an insoluble product. In the second laboratory experiment we learned that solubility is a measure of the amount of a substance, or solute, that can dissolve in a given quantity of solvent at a specified temperature. Substances with very low solubilities are referred to as insoluble. The solubilities of substances have been extensively studied by experimentation, and these compiled results have produced guidelines for predicting the solubility in ionic compounds.
Table 4.1 Solubility guidelines for common ionic compounds in water.
| Soluble Ionic Compounds | Important Exceptions | |
|---|---|---|
| Compounds containing | NO3− | None |
| CH3COO− | None | |
| Cl− | Compounds of Ag+, Hg22+, and Pb2+ | |
| Br− | Compounds of Ag+, Hg22+, and Pb2+ | |
| I− | Compounds of Ag+, Hg22+, and Pb2+ | |
| SO42− | Compounds of Sr2+, Ba2+, Hg22+, and Pb2+ | |
| Insoluble Ionic Compounds | Important Exceptions | |
| Compounds containing | S2− | Compounds of NH4+, the alkali metal cations, and Ca2+, Sr2+ and Ba2+ |
| CO32− | Compounds of NH4+, the alkali metal cations | |
| PO43− | Compounds of NH4+, the alkali metal cations | |
| OH− | Compounds of NH4+, the alkali metal cations, and Ca2+, Sr2+, and Ba2+ |
Table 4.1 allows us to predict if a precipitate will form when ions are mixed. The anions in the top section (e.g., nitrate, NO3–, acetate, CH3COO–, chloride, Cl–) form quite soluble ionic compounds. There are exceptions, however, as compounds such as AgCl, and PbCl2 are not very soluble. In contrast, the anions in the bottom section of the table (e.g., carbonates, CO32–, phosphates, PO43−, hydroxides, OH−) are usually insoluble.
Question 4.1: Use the solubility table to predict the products of the following exchange reactions. Write the net ionic equation, if applicable.
KCl(aq) + Mg(NO3)2(aq) →
BaCl2(aq) + Na2SO4(aq) →
Na2CO3(aq) + BaCl2(aq) →
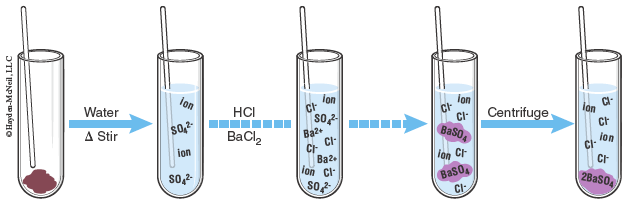
Gravimetric analysis will be used in this lab to determine the amount of a component in a mixture. Gravimetric analysis is a method of analysis in which the component is converted to another form so that it may be easily separated and weighed. Oftentimes we will convert an ion into a precipitating compound and then isolate the solid. By measuring the mass of the original sample and then the mass of the known product, the mass percent of the ion in the original sample may be calculated. In this lab, the entire procedure is carried out in a 4-mL test tube and a capillary tube will be used for stirring. Since we are working with very small masses, any change in mass will affect our results, thus, the capillary rod used for stirring will remain in the test tube the entire time and be included for any weighings.
The unknown sample contains a mixture of soluble salts and your task is to determine the amount of sulfate ions present. The first part of the lab (steps 1–3) requires you to determine the mass of the unknown sample, and you are instructed to use the same balance for the remainder of this lab.
Question 4.2: Which type of balance, top-loading or analytical, is more appropriate for use in this lab?
In the second part of the lab you will dissolve your sample (steps 4–5) and then precipitate a solid (steps 6–10). From the solubility table we can notice that sulfates are generally soluble with a few exceptions, including those with barium, thus, the precipitating agent is BaCl2. From the table we notice that BaCl2 is soluble and will dissociate into Ba2+ and Cl− ions. The Ba2+ ions are then free to bond with the sulfate ions and solid BaSO4 is formed. Once the unknown sample is dissolved in water, a drop of 3 M HCl is added to remove any CO2. Having dissolved CO2 in the tube with barium ions present could precipitate BaCO3 along with the BaSO4. Excess BaCl2 will be added to precipitate BaSO4, thus removing all of the sulfate ions.
Question 4.3: Sometimes barium compounds are not available for use in this experiment. What are two other precipitating agents that would also serve to remove the sulfate ions in the unknown sample?
Even though barium sulfate is considered insoluble, we are still able to measure if any of the substance is capable of dissolving. The solubility of BaSO4 is 0.000222 g in 100 mL of water at 18 °C. The solubility of BaSO4 is further reduced by the presence of excess barium ions.
Question 4.4: How many grams of BaSO4 will dissolve in 4 mL of water? Will the amount be measurable with our analytical balance?
Heating the precipitate in contact with the solution from which it came is called digestion and produces a more crystalline form of the precipitate. The third portion of the lab works to isolate and wash the precipitate (steps 11–14). Lastly the precipitate is dried and weighed (steps 15–18). The drying process is done in a sand bath and must be done slowly to avoid the sample ejecting from the test tube. Once the sample is dried the mass of the precipitate may be used to calculate the percent of sulfate in the original sample.
When analyzing your results, the percent sulfate of the original sample may be calculated using the expression:
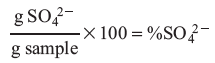
In order to perform this calculation we need to find the grams of sulfate from our original sample. In the lab we found the mass of the BaSO4 that precipitated out. Using the mole ratios from the net ionic equation, the corresponding molar masses and dimensional analysis we may convert the mass of our precipitate, BaSO4, to mass of sulfate:
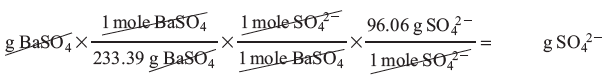
Materials Provided
Equipment
- 2 4-mL test tubes
- 2 capillary tubes
- medicine dropper
- 9-inch micropipet
- water bath (100-mL beaker)
- test tube holder
- Bunsen burner, wire gauze
- thin-walled rubber tubing
- ring stand, ring
Common Equipment
- centrifuge
- sand bath, hot plate
- analytical balance
Chemicals
- 3 M hydrochloric acid, HCl
- 10% barium chloride, BaCl2
- unknown sample
Cautions
Be careful to avoid burns from the ring, hot plate, and the open flame. Barium chloride is toxic. If any of the solution comes in contact with your skin or clothing, flood the affected area immediately with water.
Procedure
- Clean and dry two 4-mL test tubes. Mark them with a permanent marker to distinguish them from each other and also from those of other students. Place a capillary tube, closed end down, in each test tube. If the capillary tube breaks at any time, add the broken pieces to the test tube.
- Weigh each test tube on the analytical balance. All weighings for the experiment should be done on the same balance.
- Place a small amount of unknown in each test tube (about the size of two grains of rice). Reweigh each test tube. The sample should be in the range of 50 to 75 mg.
B. Dissolve the sample and add precipitating agent.
- Prepare a hot water bath using a 100-mL beaker.
- Add about 1 mL (20 drops) of distilled water to each test tube to dissolve the sample. Use the capillary tube for stirring. Warm in the hot water bath if necessary. The sample must be completely dissolved before proceeding. An additional 5 drops of water may be required.
Question 4.5: If your unknown samples were not completely dissolved, how would this affect the percent sulfate you calculate at the end of this lab?
- Add 1 drop of 3 M HCl to each test tube and stir.
- Add 1.0 to 1.5 mL of 10% BaCl2, dropwise, to each tube. Stir after each 2 or 3 drops. Caution: Try not to get BaCl2 solution on your skin. If you do, wash it off immediately with lots of water.
- Place tubes and contents in the boiling water bath for 3 to 5 minutes to digest.
- Remove the test tubes from the boiling water bath and allow to cool. Centrifuge the test tubes for one minute. Centrifuge the sample test tubes two at a time for balance—be careful not to break the capillary tubes.
- Test for complete precipitation by adding 1 drop on BaCl2 solution without disturbing the precipitate at the bottom of the tube. If no cloudiness appears, proceed to step 11. If the solution becomes cloudy, add 10 more drops of BaCl2, stirring after each 2 to 3 drops, and go back to step 8. If step 10 must be repeated more than two times, check your sample weight (step 3) with your TA.
Question 4.6: If the precipitation is not complete, the solution becomes cloudy
when one drop of BaCl2 is added. What is producing the cloudiness?
C. Isolate and wash the precipitate.
- Use a 9-inch micropipet to withdraw most of the clear supernatant liquid above the BaSO4 precipitate, being careful not to remove any solid.
- Add 10 drops of distilled water to each test tube and stir until all of the precipitate is suspended.
- Centrifuge for 30 seconds. Withdraw most of the supernatant liquid, again being careful not to remove any solid.
- Repeat the washing procedure (steps 12 and 13) two more times.
D. Dry the precipitate and heat to constant mass.
- Gently heat the test tube over an open flame to remove most of the water. Warm the test tube just above the precipitate, and then gradually move more of the precipitate into the flame. Heating the bottom of the test tube first will cause the sample to be ejected.
Question 4.7: If your sample were ejected at this point, how would this affect the percent sulfate you calculate for your unknown sample?
- Place the test tube and contents in a warm sand bath, only 1 to 2 cm deep at first. Push it further into the sand every 5 to 10 minutes. Total heating time is about 20 minutes.
- Remove the tubes from the sand bath, allow to cool, and weigh.
- Return the test tubes to a hot sand bath for at least 15 minutes. (The slow heating process should not be necessary at this time.) Cool and re-weigh. If the mass agrees with the previous value within 0.0005 g, the experiment is complete. If not, repeat this step until agreement is reached.
Question 4.8: If you did not heat your sample to a constant mass, what effect would this have on the percent sulfate you calculate?
Waste Disposal
Use the capillary tube to dislodge the BaSO4, and then rinse the test tubes with distilled water. All solutions containing barium and the BaSO4 precipitate must be collected in the inorganic salts waste container and neutralized with sodium hydroxide. The waste disposal sheet should be properly filled in. Your lab instructor will dispose of the total volume in the appropriate container. Unused unknowns may be rinsed down the drain.
Answer Clinic
Question 4.1
Use the solubility table to predict the products of the following exchange reactions. Write the net ionic equation, if applicable.
Answer
KCl(aq) + Mg(NO3)2(aq) → no reaction, no precipitate formed.
The solution contains K+, Cl–, Mg2+, and NO3– ions.
The solution contains K+, Cl–, Mg2+, and NO3– ions.
BaCl2(aq) + Na2SO4(aq):
Ba2+(aq) + SO42–(aq) → BaSO4(s)
Na2CO3(aq) + BaCl2(aq):
Ba2+(aq) + CO32–(aq) → BaCO3(s)
Question 4.2
Which type of balance, top-loading or analytical, is more appropriate for use in this lab?
Answer
The analytical balance.
Question 4.3
What are two other precipitating agents that would also serve to remove the sulfate ions in the unknown sample?
Answer
Based on the solubility table, Sr2+ and Pb2+ form insoluble compounds with sulfate. Sr(NO3)2 or Pb(NO3)2 would be reasonable choices since both of these nitrates are themselves soluble and a source of the cations.
Question 4.4
How many grams of BaSO4 will dissolve in 4 mL of water?
Answer
The solubility is 0.000222 g in 100 mL.
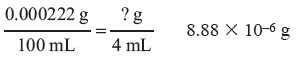
Will the amount be measurable with our analytical balance?
No, the analytical balance is only sensitive to 0.0001 g.