Chapter 1. Emission of Light: Discharge Lamps and Flame Tests
Discussion
Objectives

At the end of this activity you should be able to:
- Describe how discharge lamps emit photons following electrical excitation of gaseous atoms.
- Discuss the interdependence of scientific and technological developments. Specifically, how the technology of discharge lamps is connected to Bohr’s model of the atom and line spectra.
- Describe how thermal energy may lead to the emission of photons, and how this is accomplished in flame tests.
- Write the electron configuration for many-electron atoms in their ground state.
- Write the electron configuration of ions.
- Understand the meaning of ionization energy and how it is related to the spacing of electronic energy levels.
- Relate the concept of effective nuclear charge to the spacing of electronic energy levels for sodium and neon.
These learning objectives are assessed in the post-lab Carmen quiz.
There is no laboratory report.
Introduction
As you learned in an earlier investigation, when light is emitted from a single element (like gaseous hydrogen) and passed through a prism only a small number of discrete lines are observed. Atomic models account for this phenomenon by proposing that an electron absorbs energy and moves to an excited state. When the electron subsequently moves to a lower energy level, a photon is emitted. The reason that discrete lines are observed, and not a continuous spectrum, is because the different energy levels in the atom are quantized and have specific values. Finally, the energy that moves the electron to an excited state may be radiant, heat, or electrical. In your earlier investigation radiant energy led to the excitation. In this investigation you will consider examples of thermal (heat) and electrical excitations.
What are everyday examples of light being emitted following electrical or thermal excitation? As you will see in this investigation, technological applications like sodium, neon, or mercury vapor lamps are based on electrical excitations and the brilliant colors of fireworks (kaboom!) depend on thermal excitations. Phenomena such as these are explained by extending our conceptual models from last lab (for hydrogen) to describe polyatomic atoms and ions.
| Activity | Comment | Estimated time | |
| 1. | Computer simulation: Discharge lamps | Worksheet | About 60 minutes |
| 2. | Flame tests | Completed in lab | About 30 minutes |
| 3. | Post-Lab Carmen Quiz | Completed individually | Completed outside of lab |
| Total time ~1.5 hours |
All in-lab activities may be discussed with classmates and/or completed in a small group. The teaching assistant will help manage your time.
Part 1. Computer Simulation—Neon Lights and Discharge Lamps
After this computer simulation you should be able to 1) explain how discharge lamps function, 2) discuss the emission of photons from these lamps in terms of an atomic model, like the Bohr model, and 3) compare and contrast the emission of photons from different lamps.
There are several variables you can manipulate within the simulation:
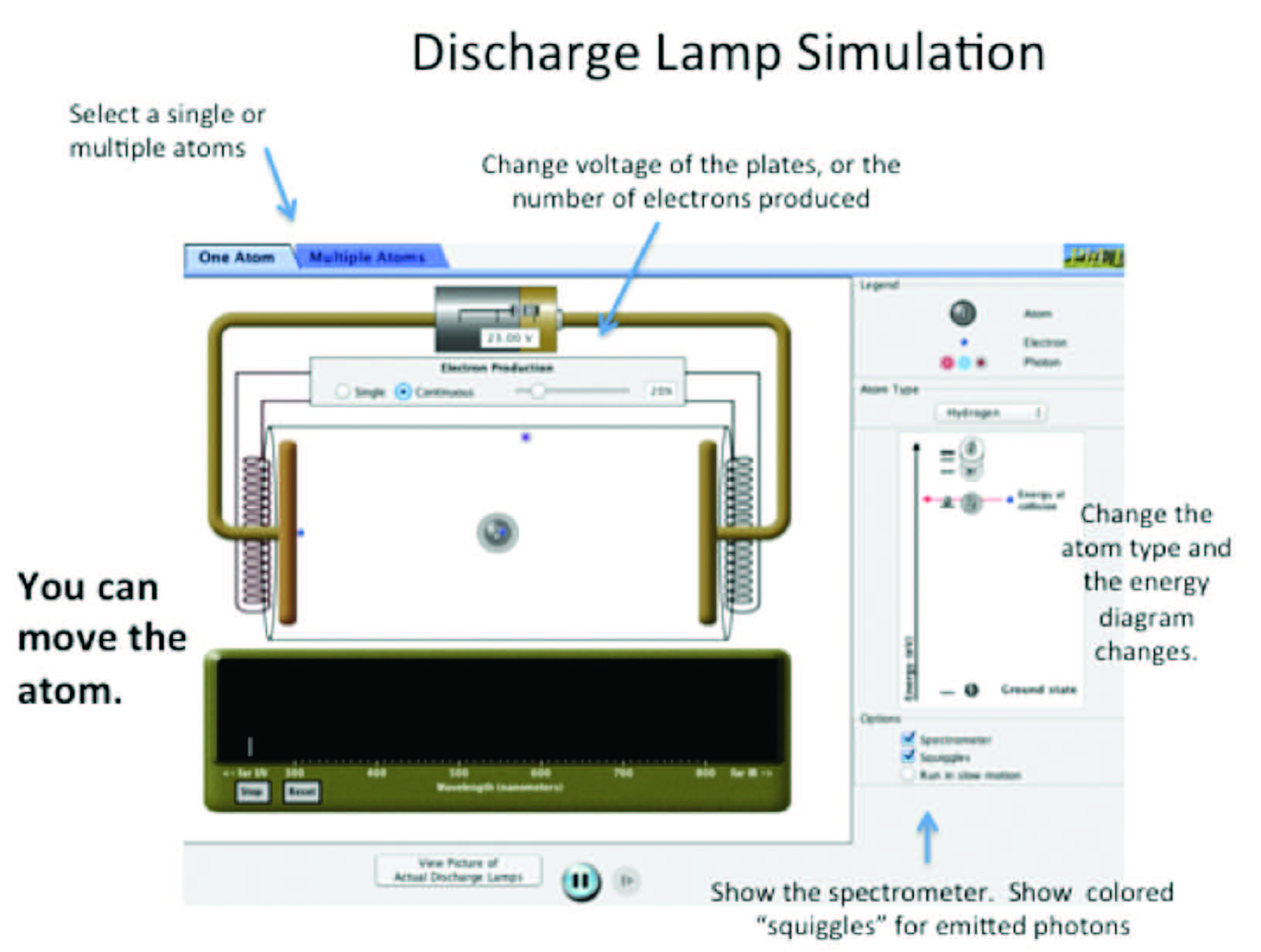
Figure 8.1 Overview of simulation options.
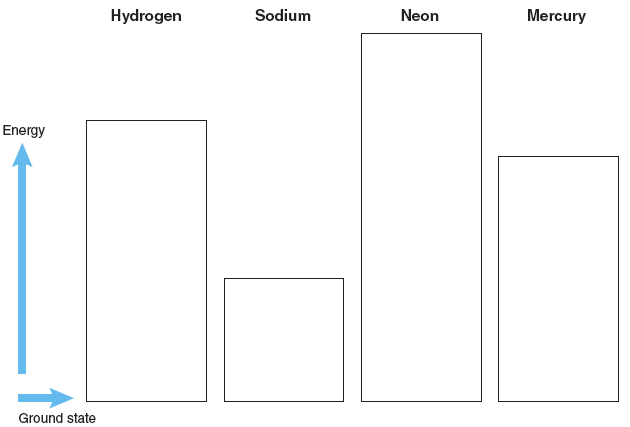
To begin your investigation, sketch the electronic energy level diagram for the different atoms. To see the electronic energy levels click on Atom Type and select the desired atom.
Sketch the electronic energy levels for each atom in the appropriate box:
What is the electron configuration for each atom in its ground state?
Hydrogen:
Sodium: [Ne]3s1
Neon:
Mercury:
What are allowed quantum numbers (n, l, ml, and ms) for the most energetic electron in the ground state? (Sodium is done as an example.)
Hydrogen:
Sodium: n = 3, l = 0, ml = 0, ms = +1/2 or –1/2
Neon:
Mercury:
To get started: By having electrons strike the atom you can cause it to emit photons, and the energy of the emitted photons is recorded by the spectrometer. The electron production can be single or continuous and the voltage may be adjusted (see the top of the screen, as shown in Figure 8.1). You must turn on the spectrometer to record the emitted photons (right side of the screen, Figure 8.1). In the “Atom Type” pull-down menu you can configure (move) the energy levels and also add energy levels, or choose a particular element (like neon).
Start producing electrons and use the simulation to gather information and answer the questions on the next page.
Select the ONE ATOM tab.
| Statement | True or False? | |
|---|---|---|
| 1 | In Bohr's model of the hydrogen atom, as the principal quantum number (n) increases, the energy levels follow a clear pattern and converge. An identical pattern is found for Hg, Ne, and Na. |
|
| 2 | The energy emitted from a discharge lamp is quantized. (Hint: look at the spectrometer.) |
|
| 3 | Photons are emitted as electrons move to a higher energy level. | |
| 4 | If the spacing between two electronic energy levels in atom A is larger than in atom B, then the wavelength of the light emitted by atom B will be longer. (You may want to use the configurable atom to answer this question.) | |
| 5 | If the spacing between two electronic energy levels in atom A is smaller than in atom B, then fewer photons will be emitted by atom B. (You may want to use the configurable atom to answer this question.) | |
| 6 | Electrons are destroyed when they strike an atom, causing an |
Can an emission spectrum be produced from a single neon atom? (The answer is “yes.”) The emission spectra for single atoms of hydrogen, mercury, or sodium occur “automatically” in this simulation. This is not the case for neon. Try to manipulate the different variables (voltage, number of electrons produced, placement of the Ne atom, etc.) and figure out how to obtain the Ne emission spectrum.
Why is it more difficult to cause photons to be emitted from neon?
Select the MULTIPLE ATOM tab.
| Statement | True or False? | |
|---|---|---|
| 1 | In a discharge lamp the energy that moves the electron to a higher energy level (an excited state) must be electrical. |
|
| 2 | The only way to emit IR photons is by having empty electronic energy levels very close to the ground state. |
|
| 3 | Neutral atoms of different elements have different number of |
|
| 4 | For a collection of many atoms, some are in an excited state and others are in the ground state. At any point in time most of the atoms have electrons in the ground state. |
1.1 Ionization Energy
In this simulation you have added energy to atoms in their gaseous state causing electrons to move to excited states. What if even more energy was added to an atom in its gaseous state? Would it be possible to completely remove an electron and form an ion? YES!
The ionization energy of an atom or ions is the amount of energy required to remove an electron from the ground state of the isolated gaseous atom or ion. For sodium, the first ionization energy is the energy required for the process:
Na(g) → Na + (g) + e–
What is the electron configuration for each ion after an electron was removed?
Hydrogen: N/A
Sodium:
Neon:
Mercury:
Consider the first ionization energies for different atoms shown in the figure.
Based on these data, how is ionization energy related to the electronic energy level diagrams shown in the simulation?
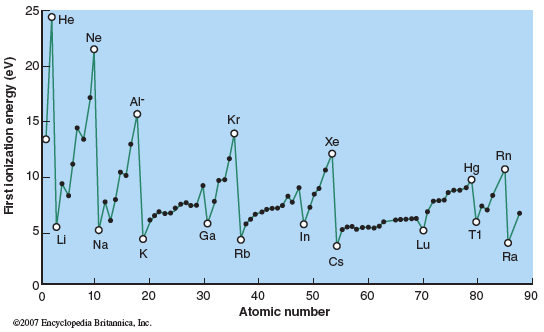
Do you predict the spacing of the electronic energy levels (from N = 1 to N = 15) will be the same or different for Li, Na, and K (moving down a column in the periodic table)?
How do you predict the spacing of the electronic energy levels (from N = 1 to N = 15) change moving from Li to O to Ne (moving across a row in the periodic table)?
The spacing of the electronic energy levels for Na and Ne are very different. Explain why. (Hint: Consider the effective nuclear charge.)
Scientific and Technological Advancements
Scientific and technological advancements are often interdependent. For example, cathode rays were generated in tubes similar to discharge lamps and investigation of these rays led to the discovery of the electron by J.J. Thompson (as discussed in Chapter 2). A similar tube was used to investigate the photoelectric effect by Philipp Lenard that led to Einstein’s interpretation of light as a particle (or “photon,” discussed in Chapter 6). These ideas (electrons and photons) were combined in Bohr’s atomic model and description of the emission of photons. Subsequently, these ideas have been used to develop new technologies, such as sodium, mercury vapor, and neon lamps. In short, this is an example of technology fueling scientific advances, which then produce technological gains.
Sodium, mercury vapor, and neon discharge lamps are commonly used in our modern society. Use your knowledge of discharge lamps and the computer simulation to answer the following questions:
One of these lamps has a great deal of heat being emitted along with visible light. Which lamp will be the hottest? (Hint: Look at the infrared region.)
The color of light we observe from a discharge lamp is a combination of all of the individual emission lines. If the energy of the photons span the entire visible region the color will appear “white.” This combining of colors is the opposite of what occurs in a spectroscope.
Which lamp emits yellow light (and is used in street lamps)?
Which lamp emits white light (and is used in warehouses, sports arenas, and factories)?
Part 2. Flame Tests of Metals
When energy is added to atoms or ions of a particular element, electrons can absorb the energy and move to a higher energy level. When the electrons of these “excited” atoms or ions return to their normal ground state, energy is emitted.
As you have observed experimentally the emission spectra for hydrogen and helium are different from each other. Also, the energy level diagrams may be different for different elements and this means their emission spectra may be different. When you look at these excited atoms or ions with the naked eye you see only a single color that is the “sum” of all the individual colors emitted. In this activity you will observe this phenomena for different metal salts that have been excited by heating in a flame.
Materials Provided
Equipment
- burner
Common Equipment
- wooden splints (pre-soaked)
Chemicals
- salts of various metals, such as sodium, potassium, calcium, barium, strontium, lithium, and copper.
Caution
Goggles must be worn throughout the experiment. Do not touch solutions with your bare hands. Use caution with the burner.
Waste Disposal
Metal salts go into the inorganic waste.
Procedure
- Light and adjust your Bunsen burner; ask for assistance if you are unsure how to use the burner.
- A test solution may be provided for each metal. If not, simply add a small amount of the provided solid salt (about ½ of a gram) to a small amount of distilled water (about 10–20 mL) to prepare a reasonably concentrated solution. Calculating the exact concentration is not necessary.
- To do a flame test with each metal salt, get a film of the solution on a wooden splint and bring it into the hottest part of the flame. To avoid contamination, use a different splint for each test solution. If this produces poor color, then try the edge of the burner flame. Repeat the test as often as necessary to see the flame test color. Be sure not to overheat the splint.
- In your notebook record the name of each compound and the overall color of each when it is put in the flame.
Periodic Table
New section content