Chapter 18. Equilibrium and Le Châtelier’s Principle
Discussion
Objectives

- Explore chemical equilibrium in the laboratory and see Le Châtelier’s principle in effect.
- Observe the effects of changes in concentration on a system in equilibrium and calculate its rate constant.
- Observe the effect of temperature on a system at equilibrium and determine if a reaction is endothermic or exothermic and properly place heat as a reactant or product in the balanced equation.
Introduction
Previously in chemistry we have learned about the process of equilibrium, a condition in which the forward and reverse reactions are occurring at equal rates. From a quick glance it may appear that no reaction is taking place because there is no net change in the reactants or products. However, this process is more appropriately referred to as dynamic equilibrium, indicating that the forward and reverse reactions are happening simultaneously.
External conditions (temperature, pressure, changing concentrations) will disrupt a chemical equilibrium and a new equilibrium will be established. In this lab we will manipulate chemical equilibria and explore Le Châtelier’s principle.
Connection to Lecture
Consider the equilibrium reaction equation shown below. In the forward reaction, A and B react to form C and D. In the reverse reaction, C and D react to form A and B. The equilibrium is indicated by writing a single equation with a forward and reverse arrow. Lowercase letters represent coefficients in the balanced equation.
In equilibrium the concentrations of all reactants and products are constant. There is a relationship between concentrations that has been shown to be a constant for a system at equilibrium. The relationship is the molar concentrations of products, raised to the power of their coefficients, divided by molar concentrations of reactants, raised to the power of their coefficients. When the system is at equilibrium, the expression is equal to a constant, the equilibrium constant, K.
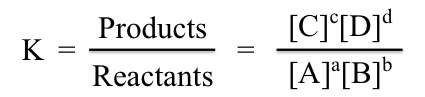
The magnitude of the equilibrium constant can vary from very large to very small. This value can provide information about the makeup of the equilibrium. Very large K values correspond to large numerators and small denominators in the equation above. This means high concentrations of products and low concentrations of reactants. Thus a large K indicates an equilibrium in which the forward reaction dominates.
Question 18.1: What does a small value of K indicate in terms of reactant and product concentrations?
There is a generalization that allows us to predict the effect of changing conditions on a system at equilibrium. It is known as Le Châtelier’s principle and it can be stated: If a stress is applied to a system at equilibrium, the system will shift, if possible, to a new position of equilibrium to counteract (minimize) the stress. There are three kinds of stress that can be brought to bear on a system at chemical equilibrium: (1) changes in concentration, (2) changes in temperature, and (3) changes in pressure. You will be observing shifts in equilibria within two different systems. The first will investigate those brought about by changes in concentration. You will observe shifts brought about by changes in temperatures in the second system.
Shifting Equilibrium by Changing Concentrations
Shifts in the equilibrium are caused by changes in concentrations and can be predicted by Le Châtelier's principle. We will investigate this by looking at the hydrolysis (reaction with water) of antimony trichloride.
The appearance and disappearance of the white solid, SbOCl, is the indication of the direction of shift.
Question 18.2: If the reaction test tube above has a large amount of white solid, does the reaction favor the reactants or products?
The equilibrium constant expression for this reaction is
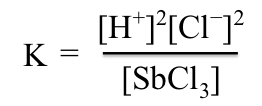
Note the omission of H2O and SbOCl. Concentrations of pure liquids and pure solids do not appear in an equilibrium constant expression. Molar concentrations of pure liquids or solids are constant. The small amounts of HCl and SbCl3 do not change the concentration of water appreciably. Likewise, SbOCl is a solid with a characteristic density; whether a large amount or a small amount of SbOCl is present, the concentration is constant. Some solid must be present if the equilibrium is established, but the amount does not matter. Concentrations of pure solids and pure liquids are therefore not incorporated into the equilibrium constant.
Concentrations of H+, Cl−, and SbCl3 at equilibrium are needed to calculate the equilibrium constant for this reaction. They are calculated from starting amounts and amount of water added. Water is added until white solid SbOCl persists, indicating that equilibrium has been established.
The solution of antimony trichloride contains 0.50 M SbCl3 and 6.0 M HCl. Addition of water to this solution results in dilution; the number of moles of SbCl3, H+, and Cl− are not changed.
Moles are calculated from volume and molarity.
Note that V2 is the total volume of the solution, the original volume plus the volume added. Contrarily, when 6 M HCl is added to the dilute solution of antimony trichloride in HCl, the moles of H+ and Cl− will change. These must be combined before dividing by the total volume to get [H+] and [Cl−]. The [SbCl3] is still simply found using a dilution calculation.
In Part B the [H+], [Cl−] and [SbCl3], are found using simple dilution calculations. Once these are known, the K can then be calculated from the K expression on the previous slide.
Shifting Equilibrium by Changing Temperature
The second system you will study involves a hexaaquacobalt(II)–tetrachlorocobalt(II) equilibrium. The reaction is shown below.
Shifts in this equilibrium are detected by color change between the pink Co(H2O)62+ and the blue CoCl42−. Shifts are caused by additions of concentrated hydrochloric acid and additions of water.
Question 18.3: Would you predict the addition of Cl− to cause the color to turn towards blue or to turn towards pink?
Hydrochloric acid increases the concentration of the chloride ion in the solution and the expected shift is observed. Addition of water to the solution containing the cobalt(II) ion and HCl also causes a shift, but not because of an increase in the concentration of water. Since water is the solvent, the concentration is relatively high (55.5 M) and nearly constant—adding more water does not change the concentration of the water. The effect of adding water is to decrease the concentration of the ions in solution. Note that this same argument applies to the antimony trichloride system when water is added.
An additional stress that is brought to bear on the cobalt(II) system is a change in temperature. An increase in temperature causes a stress of added heat and a decrease in temperature removes heat. The observed color changes will tell you the direction of shift that each change in temperature causes. The shift will be in the direction that minimizes the stress. The reaction that occurs when heat is added will be the reaction that uses heat—the endothermic direction. If heat is consumed in the forward direction, ΔH is positive; if heat is evolved in the forward direction (consumed in reverse), ΔH is negative.
The concentrations of the chloride ion in this equilibrium may be calculated. It is fi rst necessary to calculate the molarity of concentrated hydrochloric acid. This solution has a density of 1.185 g/mL and contains 37.0% HCl by weight, thus concentrated HCl is 12.0 M. A medicine dropper will deliver approximately 20 drops to give one mL of concentrated HCl.
Materials Required
Equipment
- three 25-mL test tubes
- 75-mL test tube
- 10-mL graduated cylinder
- 5-mL pipet
- 25-mL buret, clamp
- 125-mL Erlenmeyer flask
- medicine dropper
- Bunsen burner, wire gauze
- thin-walled rubber tubing
- ring stand, ring
Chemicals
- antimony trichloride, 0.50 M SbCl3 in 6 M HCl
- solid cobalt(II) nitrate hexahydrate, Co(NO3)2· 6 H2O
- solid cobalt(II) chloride hexahydrate, CoCl2· 6 H2O
- 0.4 M Co(NO3)2, aqueous
- 0.4 M Co(NO3)2, in absolute ethanol
- absolute ethanol, C2H5OH
- 6 M hydrochloric acid, HCl
- concentrated hydrochloric acid, HCl
- ice
Common Equipment
- none
Cautions
Be careful to avoid burns from the ring, beaker, and the open flame. Antimony compounds are toxic and irritating to the skin. Concentrated hydrochloric acid causes severe burns. Wash your hands well with soap and water. Goggles must be worn at all times.
Procedure
Discussions with your peers and TA are encouraged as you proceed.
- Add 5.0 mL of the antimony trichloride solution to a clean, dry 75-mL test tube using a 10-mL graduated cylinder. Record the appearance of the solution and the initial concentrations of SbCl3 and HCl in your notebook.
- Add 4.0 mL of distilled water to the test tube. Record the appearance before and after shaking the test tube. Calculate the concentrations of antimony trichloride and hydrochloric acid, assuming dilution only.
- Repeat step 2 three times to give a final volume of 16 mL of distilled water added.
- Add 2.0 mL of 6 M HCl to the test tube. Record the appearance before and after shaking the test tube. Calculate the concentration of antimony trichloride and hydrochloric acid, assuming dilution only.
- Add 5.00 mL of fresh antimony trichloride solution to a clean, dry 75-mL test tube using a 5-mL pipet. Record the appearance of the solution and the initial concentration of SbCl3 and HCl in your notebook.
- Clean a buret with soap and water. Rinse well with distilled water. Fill the buret with distilled water and check for bubbles in the tip.
- Record the initial buret reading in your notebook. Titrate the SbCl3 solution with distilled water until a slightly milky appearance persists after thorough mixing. Record the final buret reading.
- Calculate the concentrations of antimony trichloride and hydrochloric acid assuming dilution only. Calculate the equilibrium constant, K.
C. Observation of the hexaaquacobalt(II)-tetrachlorocobalt(II) equilibrium.
- Your teaching assistant will have two trays on the front desk containing cobalt(II) nitrate hexahydrate and cobalt(II) chloride hexahydrate. Use this material to perform steps 10 through 12.
- Record the color of the solids: cobalt(II) nitrate hexahydrate and cobalt(II) chloride hexahydrate.
- Add a few crystals of each salt to separate dry 25-mL test tubes. Add 5 mL of distilled water and record the colors.
- Add a few crystals of each salt to separate dry 25-mL test tubes. Add 5 mL of absolute ethanol and record the colors.
- Add 5.0 mL of 0.4 M aqueous Co(NO3)2 to a clean, dry 75-mL test tube. Add 8.0 mL of concentrated HCl to the test tube and swirl the test tube. Set this tube aside to be used as a reference.
- Add 5.0 mL of 0.4 M aqueous Co(NO3)2 to a second clean, dry 75-mL test tube.
- Add 2.0 mL of concentrated HCl to the test tube. Record the color after swirling the test tube. Calculate the concentration of chloride ion, assuming dilution only.
- Repeat step 15 three times to give a volume of 8 mL of concentrated HCl added. Compare the color to the color of the solution from step 13—it should be the same.
- Repeat step 15 four times to give a final volume of 16 mL of concentrated HCl added. Each time, compare the color to the color of the solution from step 13.
- Add 4.0 mL of distilled water to the test tube from step 17. Record the color after swirling the test tube. Calculate the concentration of chloride ion, assuming dilution only.
- Repeat step 18 three times to give a final volume of 16 mL of distilled water added.
- Add 5.0 mL of 0.4 M Co(NO3)2 in absolute ethanol to a clean, dry 25-mL test tube.
- Add concentrated HCl to the test tube using a medicine dropper. Swirl the test tube after each drop. Record the number of drops required to give the same color as was observed for the aqueous cobalt(II) nitrate in step 16 after addition of 8 mL of HCl. This should be the color of the solution prepared and set aside in step 13.
- Continue adding concentrated HCl to the test tube from step 21 using a medicine dropper. Swirl the test tube after each drop. Record the number of drops required to give a distinctly blue solution. Calculate the concentration of chloride ion in the blue solution, assuming dilution only.
- Pour the solution prepared in step 13 into a clean, dry 125-mL Erlenmeyer flask. Add 4.0 mL of distilled water to the flask, and mix thoroughly by swirling the flask. If the color is not violet, adjust it accordingly by adding drops of distilled water or concentrated HCl.
- Divide the solution equally into three clean, dry 25-mL test tubes. Place one tube into an ice water bath, keep one at room temperature, and place one tube into a boiling water bath. Record the colors of the solutions.
- Exchange the test tubes between the ice bath and boiling water bath, and record the colors. Note how the color changes as a function of temperature change.
Waste Disposal
All solution containing antimony(III) and cobalt(II) must be collected in a beaker at your desk. Neutralize the solution with 6 M NaOH while stirring. The solution will be neutral when a white precipitate of SbOCl persists. Dispose of the total volume in the inorganic waste container. Sign the waste disposal sheet and fill in the volume.
Points to Consider
In this lab you are exploring Le Châtelier’s principle and (1) how concentrations affect a system and (2) how temperature affects a separate system.
- For this lab include all of your observations and data in the report sheets and turn them in with your lab report; show a sample of dilution calculation and sample K calculation. Answer the questions given on the report sheet.
- For the antimony trichloride system, what happened when water was added? What happened when 6 M HCl was added?
- What was the K for this system?
- For the cobalt(II) system, what happened when concentrated HCl was added? What happened when water was added?
- Explain the colors observed for the cobalt(II) nitrate and cobalt(II) chloride in water and absolute ethanol.
- What is the temperature effect in the cobalt(II) equilibrium? Is this reversible? Is the system endothermic or exothermic?